
���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�á���ͼ��ʾһ�����أ�װ�е��Һa��X��Y������缫�壬ͨ��������ֱ����Դ��������ش��������⣺
(1)��X��Y���Ƕ��Ե缫��a�DZ���NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸�η�̪��Һ����
�ٵ�����X�缫�ϵĵ缫��ӦʽΪ_________________________��X�缫�����۲쵽��������___________________________��Y�缫�ϵĵ缫��ӦʽΪ____________________����õ缫��Ӧ����ķ�����___________________
(2)���õ�ⷽ��������ͭ�����Һaѡ��CuSO4��Һ����
��X�缫�IJ�����________���缫��ӦʽΪ__________________��Y�缫�IJ�����________���缫��ӦʽΪ________(˵�������ʷ����ĵ缫��Ӧ����д��)��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����(Na2CO3)�����������о��й㷺����;��������ʵ����ģ���Ƽ� ԭ����ȡNa2CO3������ͼ��
ԭ����ȡNa2CO3������ͼ��

��֪����ʳ��ˮ��ͨ��NH3��CO2�����ķ�ӦΪNaCl��NH3��CO2��H2O===NaHCO3����NH4Cl����ش��������⣺
(1)�����к��е�����������Ca2����Mg2����SO42���ȡ�
���Ƴ��ӵIJ���˳����a��________��________��________��b(����ĸ���)��
a�������ܽ⣬��ȥ���� b�������� ���pHc������Ba(OH)2��Һ d������Na2CO3��Һ
���pHc������Ba(OH)2��Һ d������Na2CO3��Һ
e������
��ʳ��ˮ����ͨ��NH3����ͨ��CO2��������_________________________ _________________________________________________________________��
(2)���չ���A��Na2CO3��________(����ĸ���)�н��С�
a������ b�������� c���ձ� d����ƿ
֤����ҺA�к���NH4���ķ�����_____________________________________ _________________________________________________________________��
����ҺA�����ؽᾧ�ܹ����NH4HCO3����pH��13��Na����K������Һ�м�������NH4HCO3ʹpH���ͣ���Ӧ�����ӷ���ʽΪ____________________��
(3)��ͼװ���г�����ʵ�����Ʊ�CO2����________(����ĸ���)����bװ���Ʊ�NH3����Һ©����ʢ�ŵ��Լ���________(���Լ�����)����ƿ�ڿɼ���Ĺ����Լ���________(���Լ�����)��

(4)һ����Ȼ���ɷ���aNa2CO3��bNa2SO4��cH2O��ijͬѧ���������ṩ���Լ�����������¼����ⶨNa2CO3������������ʵ�鷽����(������ѡ)���ʵ�鷽����ȫ��
��ѡ����Լ���1.0 mol��L��1 H2SO4 ��Һ��1.0 mol��L��1 BaCl2��Һ��ϡ��ˮ����ʯ�ҡ�Ca(OH)2��Һ������ˮ
��Һ��1.0 mol��L��1 BaCl2��Һ��ϡ��ˮ����ʯ�ҡ�Ca(OH)2��Һ������ˮ
�ٳ�ȡm1g��Ȼ�����Ʒ��������������ˮ�С�
��_______________________________________________________________��
��_______________________________________________________________��
�ܼ�����Ȼ����к�Na2CO3������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������˵����Ԫ�ر���Ԫ�طǽ�����ǿ���� �� ��
��HCl��H2S�ȶ�;��HClO4���Ա�H2SO4ǿ;��Cl2����H2S��Ӧ����S;��Clԭ���������7�����ӣ�Sԭ���������6������;��Cl2��Fe��Ӧ����FeCl3��S��Fe��Ӧ����FeS;��������ǿ��,��������������
A���٢ڢۢܢݢ� B���٢ڢۢܢ� C���٢ڢۢ� D���ڢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��C(s)�� O2(g)===CO(g) ��H����110.5 kJ·mol��1��
O2(g)===CO(g) ��H����110.5 kJ·mol��1��
C(s)��O2(g)===CO2(g)����H����393.51 kJ·mol��1��
���㷴ӦC(s)��CO2(g)===2CO(g)�ķ�Ӧ�Ȧ�H��ֵΪ
A����283.01 kJ·mol��1 B����172.51 kJ·mol��1 C����283.1 kJ·mol��1 D����504.00 kJ·mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ�ǵ��CuCl2��Һ��װ�ã�����c��dΪʯī�缫���������йص��ж���ȷ����
A a������b���� B a������b����
C �������У�d�缫�������� D �������У�������Ũ�Ȳ���

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з��ӻ�������ָ���ķ�ɢϵ���ܴ��������һ����
A��ʹ��̪���ɫ����Һ��Na����Ba2����Cl—��NO3��
B�������� C2H6��CO2��SO2��NO
C���Ȼ�����Һ�� H+��K+��NO3����I—
D�����������Һ�� H+��Na+��SO42-�������Ƿ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʳ����Ҫ�ɷ���NaCl��������SO42-�������������������ӡ�����˵������ȷ����
A����ȥSO42- ����ʵ��Լ���BaCl2
B����ҵ��ͨ������Ȼ�����Һ�Ʊ������ƺ�����
C�������£�AgCl��ˮ�е��ܽ�ȴ�����ʳ��ˮ�е��ܽ��
D���÷�̪��Һ�ɼ��𱥺�ʳ��ˮ�ͱ��ʹ�����Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������Һ�У���������һ���ܹ������������(����)
A����ʹ�㷺pH��ֽ����ɫ����Һ��K����Ba2����Cl����Br��
B��������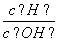 ��1012����Һ��Fe2����Mg2����NO
��1012����Һ��Fe2����Mg2����NO ��Cl��
��Cl��
C�����д���Al3������Һ��Na����Cl����AlO ��OH��
��OH��
D����ʹ���۵⻯����ֽ����ɫ����Һ��K����SO ��S2����SO
��S2����SO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼ��ʾװ�����ӣ�X��Y��Ϊ���Ե缫����ش��������⣺
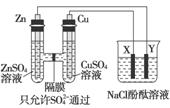
(1)ZnΪ________����
(2)���Ӻ�װ�ú��ձ��е���Һ������Ӧ�����ӷ���ʽ��___________��
(3)ͼ��ͨ����Ĥ��SO ��________(����ҡ�����)Ǩ�ƣ�Y�����丽�����ֵ�������________��
��________(����ҡ�����)Ǩ�ƣ�Y�����丽�����ֵ�������________��
(4)�����£���Zn����������32.5 gʱ��X����������8.4 L(��״��)������ʱ�ձ�����Һ�����Ϊ500 mL�����ʱ�ձ�����Һ��pH��________(���������ɵ���������ˮ)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com