
 ������±�ᷴӦ���Ʊ�±��������Ҫ������ʵ�����Ʊ�1һ�嶡��ķ�Ӧ��ʵ��װ����ͼ��
������±�ᷴӦ���Ʊ�±��������Ҫ������ʵ�����Ʊ�1һ�嶡��ķ�Ӧ��ʵ��װ����ͼ��| �۵�/�� | �е�/�� | �ܶ�/g•cm-3 | |
| ������ | -89.53 | 117.25 | 0.81 |
| 1-�嶡�� | -112.4 | 101.6 | 1.28 |
���� ��1���������ṹ��֪AΪֱ�������ܣ���Ӧ����ʱ���¶Ȳ��˳���100�棬��ȡˮԡ���ȣ����Ƭ���Է�ֹ����ʱ���У��廯�⼫������ˮ��������
��2������Ũ����Ĵ�������ˮ����ϩ���ѣ�Br-��Ũ��������ΪBr2�ȣ�Ũ�������ջ���ȣ�����ʹHBr�ӷ���
��3����ȡ������õ�1-�嶡�飬����Ҫ���������ƾ��Ƽ�������ƿ��
��4����������¶ȸ���101.6�棬����117.25�棬���¶���ˮ���γ�ˮ������
��5��1-�嶡�鲻����ˮ���ܶȱ�ˮ��
��6����������������1-�嶡������۲���������=$\frac{ʵ�ʲ���}{���۲���}$��100%��
��� �⣺��1���������ṹ��֪AΪֱ�������ܣ���Ӧ����ʱ���¶Ȳ��˳���100�棬�Ϻõļ��ȷ����Dz�ȡˮԡ���ȣ��Ϻõļ��ȷ��������Ƭ���Է�ֹ����ʱ���У��廯�⼫������ˮ��װ���е���©���������ǣ�
�ʴ�Ϊ��ֱ�������ܣ�ˮԡ���ȣ���ֹ���У���ֹ�廯�⼫������ˮ��������
��2��a��Ũ�����1-������Ӧ������ȥ��Ӧ����ϩ�������Ӽ���ˮ��Ӧ�����ѣ�ϡ�ͺ��ܷ������Ʒ�Ӧ���ٸ�����ϩ���ѵ����ɣ���a��ȷ��
b��Ũ�������ǿ�������ܽ�����������Ϊ�嵥�ʣ�ϡ��Ũ������ܼ���Br2�����ɣ���b��ȷ��
c����Ӧ��Ҫ�廯���1-������Ӧ��Ũ�����ܽ���Һ�¶����ߣ�ʹ�廯��ӷ���ϡ�ͺ����HBr�Ļӷ�����c��ȷ��
d��ˮ�Dz��ﲻ�Ƿ�Ӧ�Ĵ�������d����
��ѡ��abc��
��3����ȡ������õ�1-�嶡�飬����װ�ó����õ������ܡ��¶ȼơ�ţ�ǹܡ���ƿ������Ҫ�IJ��������Ǿƾ��ơ�������ƿ��
�ʴ�Ϊ���ƾ��ơ�������ƿ��
��4����������¶ȸ���101.6�棬����117.25�棬���¶���ˮ���γ�ˮ���������ܺ��е�������Ҫ��ˮ��
�ʴ�Ϊ��ˮ��
��5��1-�嶡�鲻������ˮ�����ܶȴ���ˮ�����Խ�1-�嶡��ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã��������²㣬
�ʴ�Ϊ���²㣻
��6��7.4g�������淴Ӧ����1-�嶡������Ϊm����
C4H9-OH+HBr$\stackrel{��}{��}$C4H9-Br+H2O
74 137
7.4g 13.7
�����Ƶ�1һ�嶡��9.6g����1-�嶡��IJ���=$\frac{9.6g}{13.7g}$��100%��70%��
�ʴ�Ϊ��70%��
���� ���⿼���л����Ʊ�ʵ�飬�漰��װ��������ķ������ۡ�ʵ�������������ԭ���ķ������ۡ����ʼ���ȣ�ע�����Ŀ��Ϣ�����ݵ�Ӧ�ã��ϺõĿ�����ѧ���������⡢���������������Ѷ��еȣ�


 �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д� Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����������Һ��ϡ���ᷴӦ��Ba2++SO42-+H++OH-=BaSO4��+H2O | |
| B�� | ��̼��þ�еμ�ϡ���CO32-+2H+=CO2��+H2O | |
| C�� | ������ʯ��ˮ��ͨ�����������̼��Ca2++2OH-+CO2=CaCO3��+H2O | |
| D�� | ��С�մ�����θ����ࣺHCO3-+H+=CO2��+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ��AԪ�صĵ縺�Դ��ϵ�����С������AԪ�صĵ縺�Դ��ϵ��������� | |
| B�� | ���������Ų�Ϊns2np6����ֻ��K��ʱΪ1s2����ԭ�ӣ���һ�����ܽϴ� | |
| C�� | ̼ԭ����1s22s22p2ת����1s22s12p3����һ�������ͷ����� | |
| D�� | NaH�Ĵ�����֧�ֿɽ���Ԫ�ط��ڢ�A�Ĺ۵� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
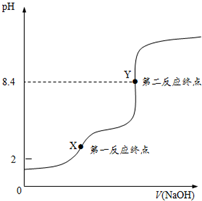 H2C2O4•2H2O�����ᣬ��Ԫ���ᣩ���ڱ���������ؾ�����Ϊ�궨NaOH��ҺŨ�ȵĻ����ʣ��Ӷ����NaOH����Һ��
H2C2O4•2H2O�����ᣬ��Ԫ���ᣩ���ڱ���������ؾ�����Ϊ�궨NaOH��ҺŨ�ȵĻ����ʣ��Ӷ����NaOH����Һ��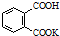 ��Ħ������Ϊ204g•mol-1��������ˮ�Ĺ�
��Ħ������Ϊ204g•mol-1��������ˮ�Ĺ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CnH2n+2 | B�� | CnH2n | C�� | CnH2n-2 | D�� | CnH2n-1Br |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com