
ijʵ��С�����ѧ�α��п����������ķ�Ӧ�������о�,�ܽ��������������H2�ķ�Ӧ:��Zn+����;��Na+ˮ;��Al+NaOH��Һ��Ϊ��ȼ����������Ӧ���ɵ�H2,�������������ͼ��ʾ��װ��ͼ:
![]()
]��ش���������:
(1)д��Al��NaOH��Һ��Ӧ�����ӷ���ʽ_______________________________��
(2)�ڵ�ȼH2֮ǰ�����Ƚ���____________________________________________��
(3)ʵ��С���ڵ�ȼ������װ���Ƶõ�H2ʱ,�٢�ʵ���óɹ�,��ȴʧ���ˡ����Ƿ�����Ϊʧ�ܵ�ԭ����Na��H2O�ķ�Ӧ����̫��,Na��������̫�١����������������Ƶ�����,�ɽ�ʦ˵̫Σ��,����Ϊ����Σ�յ�ԭ����___________________________��
(4)ʵ��С������ơ���(һ�ֲ�����ˮ��Һ̬�л���)��ˮ���ܶȷֱ�Ϊ0.97 g��mL-1��0.88 g��mL-1��1.00 g��mL-1,���ݴ˶�ʵ������˸Ľ����ڸĽ����ʵ����H2����������______________________��(��������ӿ족)
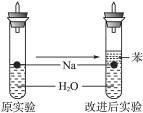
(5)2.3 g��Ͷ��20 mLˮ����ȫ��Ӧ�ų��������ڱ�״���µ������_____________,������Һ�����ʵ���Ũ����______________________��(������Һ����ı仯)
(1)2Al+2H2O+2NaOH====2NaAlO2+3H2��(2)�鴿
(3)�϶������ˮ��Ӧ�ų���������,ʹ�Թ���H2��O2�Ļ������ȼ����ը
(4)����(5)1.12 L 5 mol��L-1
��ȼ�������ڵ�ȼǰ�����鴿,���ⱬը�����Թ��в�Ҫ�������Na,����϶������ˮ��Ӧ�ų���������,ʹ�Թ���H2��O2�Ļ������ȼ����ը;�ƿ����ḽ�ű�,���ԸĽ����ʵ����H2���������ʼ���;2Na+2H2O====2NaOH+H2��,0.1 mol Na��Ӧ�ų�����Ϊ1.12 L;������Һ�����ʵ���Ũ��=![]() ��
��


 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
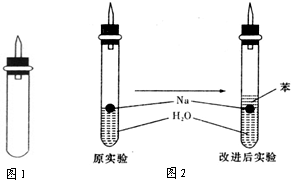 ijʵ��С�����ѧ�α��п����������ķ�Ӧ�������о����ܽ��������������H2�ķ�Ӧ����Zn+�����Na+ˮ����Al+NaOH��Һ��Ϊ��ȼ����������Ӧ���ɵ�H2�������������ͼ��ʾ��װ��ͼ��
ijʵ��С�����ѧ�α��п����������ķ�Ӧ�������о����ܽ��������������H2�ķ�Ӧ����Zn+�����Na+ˮ����Al+NaOH��Һ��Ϊ��ȼ����������Ӧ���ɵ�H2�������������ͼ��ʾ��װ��ͼ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ijʵ��С�����ѧ�α����Ѿ�ѧ�������������ķ�Ӧ�������о���д�����������������������ķ�Ӧ��
ijʵ��С�����ѧ�α����Ѿ�ѧ�������������ķ�Ӧ�������о���д�����������������������ķ�Ӧ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
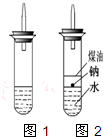
ijʵ��С�����ѧ�α��п����������ķ�Ӧ�������о����ܽ��������������H2�ķ�Ӧ����Zn�����ᡡ��Na��ˮ����Al��NaOH��Һ��Ϊ��ȼ����������Ӧ���ɵ�H2���������������װ��(ͼ1)����ش��������⣺
(1)д��Na��H2O��Ӧ�Ļ�ѧ����ʽ_____________________________________��
(2)�ڵ�ȼH2ǰ�����Ƚ���_____________________________________________��
������ _______________________________________________________��
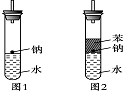 (3)ʵ��С���ڵ�ȼ��ͼ1װ���Ƶõ�H2ʱ���٢�ʵ���óɹ�����ȴʧ���ˡ����Ƿ�����Ϊʧ�ܵ�ԭ����Na��H2O�ķ�Ӧ����̫�죬Na������̫�١����������������Ƶ�����������ʦ˵̫Σ�գ�����Ϊ����Σ�յ�ԭ����________________________________________________________________________��
(3)ʵ��С���ڵ�ȼ��ͼ1װ���Ƶõ�H2ʱ���٢�ʵ���óɹ�����ȴʧ���ˡ����Ƿ�����Ϊʧ�ܵ�ԭ����Na��H2O�ķ�Ӧ����̫�죬Na������̫�١����������������Ƶ�����������ʦ˵̫Σ�գ�����Ϊ����Σ�յ�ԭ����________________________________________________________________________��
(4)ʵ��С������ơ�����ˮ���ܶȷֱ�Ϊ0.97 g��mL��1��0.88 g��mL��1��1.00 g��mL��1�����ݴ˶�ʵ������˸Ľ�(��ͼ2)���ڸĽ����ʵ����H2���������ʼ�����ԭ����________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ�߿�һ�ָ�ϰ�ο��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
ijʵ��С�����ѧ�α��п����������ķ�Ӧ�������о����ܽ���ĸ���������H2�ķ�Ӧ����Zn+���� ��Na+ˮ ��Al+NaOH��Һ ��Na+��ˮ�Ҵ���Ϊ��ȼ�����ĸ���Ӧ���ɵ�H2���������������װ��ͼ��
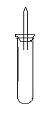
��ش��������⣺
��д��Na��H2O��Ӧ�Ļ�ѧ����ʽ ��
���ڵ�ȼH2֮ǰ�����Ƚ��� ��������
��
��ʵ��С���ڵ�ȼ������װ���Ƶõ�H2ʱ���٢ۢ�ʵ���óɹ�����ȴ
ʧ���ˡ����Ƿ�����Ϊʧ�ܵ�ԭ����Na��H2O�ķ�Ӧ����̫�죬Na��
����̫�١����������������Ƶ�����������ʦ˵̫Σ�գ�����Ϊ����Σ
�յ�ԭ���� ��
��ʵ��С������ơ�����ˮ���ܶȷֱ�Ϊ0.97g/mL��0.88g/mL��1.00g/mL�����ݴ˶�ʵ������˸Ľ���
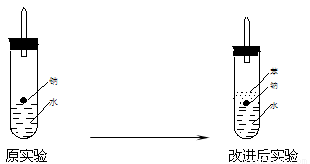
�ڸĽ����ʵ����H2���������ʼ�����ԭ����
��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com