
����Ŀ���ѿ�ҵ�е��������Է�ˮ����Ti��Fe��Ԫ�أ����ۺ��������£�

��֪��TiO2+��ˮ�⣬ֻ�ܴ�����ǿ������Һ�С�
��1��TiO2+�ѵĻ��ϼ�Ϊ________________________��
��2��������м����ѿ��ˮ�м�����м�Ƿ��������Լ���________________________��
��3������a������Ũ������ȴ�ᾧ��______________________________________________��
��4��������з�����Ӧ�Ļ�ѧ����ʽΪ________________________����Ӧ�¶�һ���������35�����£���Ŀ����____________________________________��
��5����֪Ksp=[Fe��OH��2] = 8��10-16��������У�FeCO3�ﵽ�ܽ�ƽ��ʱ���������²����Һ��pHΪ8.5�� c��Fe2+�� = 1��10-6mol/L�����ж����õ�FeCO3��________����С���û�С���Fe��OH��2��������У�Ϊ�˵õ���Ϊ������Fe2O3�������ʵ����¶��⣬����Ҫ��ȡ�Ĵ�ʩ��_________________��
��6������TiO2+��Һ���м���Na2CO3��ĩ�õ�����TiO2 nH2O������ԭ���ͻ�ѧ���������ԭ��_________________________________��
���𰸡�+4 KSCN��Һ ���ˡ�ϴ�ӡ����� FeSO4+2NH4HCO3=FeCO3��+��NH4��2SO4+H2O+CO2�� �����¶ȹ���̼����立ֽ⣬����Fe2+��ˮ������� û�� ��Ӧ�����в��������Ŀ��� ��Һ�д���ˮ��ƽ��TiO2++��n+1��H2OTiO2nH2O+2H+�������Na2CO3��ĩ��H+��Ӧ����������Һ��c��H+�����ٽ�ˮ��ƽ��������TiO2nH2O�ķ����ƶ�
��������
�ѿ�ҵ�е����Է�ˮ����Ti��Fe��Ԫ�أ�����TiO2+��Fe2+��Fe3+�����ӣ��������ۻ�ԭ�����ӵõ��������ӣ�ͨ������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����õ������������壬���Ƴ���Һ������ҺpH5-5.8��Χ�ڣ���������̼����泥����˵õ�̼�����������������������յõ������������˺�õ�������TiO2+��Һ������Na2CO3��ĩ��H+��Ӧ����������Һ��c��H+����ʹƽ��TiO2++��n+1��H2OTiO2n H2O+2H+������TiO2n H2O�ķ����ƶ����ɵõ�TiO2�ֲ�Ʒ���Դ˽�������
��1�������и�Ԫ�ػ��ϼ۴����͵�������������������㣬OԪ��Ϊ-2�ۣ�
��2������I�м����ѿ��ˮ�м�����м�Ƿ��������Լ��Ǽ����Ƿ������ӣ�
��3����Һ�еõ�������������ķ���������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����
��4��������з�����Ӧ������������̼����立�Ӧ����̼����������������李�������̼��ˮ����Ӧ�¶�һ���������35�����£���Ϊ�˱����¶ȹ���̼����立ֽ⣬����Fe2+��ˮ���������
��5��������Һ��c��Fe2+��c2��OH-����Ksp[Fe��OH��2]�Ƚϣ����ж�����Fe��OH��2��������֤������������Ϊ������Ҫ��ͨ�������������
��6��������TiO2+��Һ������Na2CO3��ĩ��H+��Ӧ����������Һ��c��H+����ʹƽ��TiO2++��n+1��H2OTiO2n H2O+2H+������TiO2nH2O�ķ����ƶ���
��1�������и�Ԫ�ػ��ϼ۴����͵�������������������㣬OԪ��Ϊ-2�ۣ���TiO2+�ѵĻ��ϼ�Ϊ+4�ۣ�
�ʴ�Ϊ��+4��
��2������I�м����ѿ��ˮ�м�����м�Ƿ��������Լ��Ǽ����Ƿ������ӣ�����ѡ�����KSCN��Һ����Һ���ɫ֤�������벻�㣬����Һ�����ɫ֤�����ۼ���������
�ʴ�Ϊ��KSCN��Һ��
��3������a����Һ�еõ������������壬����������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����
�ʴ�Ϊ�����ˡ�ϴ�ӡ�������
��4��������з�����Ӧ������������̼����立�Ӧ����̼����������������李�������̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ��FeSO4+2NH4HCO3=FeCO3��+��NH4��2SO4+H2O+CO2������Ӧ�¶�һ���������35�����£���Ϊ�˱����¶ȹ���̼����立ֽ⣬����Fe2+��ˮ���������
�ʴ�Ϊ��FeSO4+2NH4HCO3=FeCO3��+��NH4��2SO4+H2O+CO2���������¶ȹ���̼����立ֽ⣬����Fe2+��ˮ���������
��5�������²����Һ��pHΪ8.5��c��OH-��=1014��108.5=1��10-5.5�����������ݿ�֪����Һ��c��Fe2+��c2��OH-��=1��10-6����1��10-5.5��2=1��10-17��Ksp[Fe��OH��2]=8��10-16������Fe��OH��2�������ɣ�������У�Ϊ�˵õ���Ϊ������Fe2O3�������ʵ����¶��⣬����Ҫ��ȡ�Ĵ�ʩ����Ӧ�����в��������Ŀ�����
�ʴ�Ϊ��û�У���Ӧ�����в��������Ŀ�����
��6��������TiO2+��Һ������Na2CO3��ĩ��H+��Ӧ����������Һ��c��H+����ʹƽ��TiO2++��n+1��H2OTiO2nH2O+2H+������TiO2n H2O�ķ����ƶ����ɵõ�TiO2�ֲ�Ʒ��
�ʴ�Ϊ����Һ�д���ˮ��ƽ��TiO2++��n+1��H2OTiO2nH2O+2H+�������Na2CO3��ĩ��H+��Ӧ����������Һ��c��H+�����ٽ�ˮ��ƽ��������TiO2nH2O�ķ����ƶ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��![]() ��
��![]() �������£���ijŨ�ȵ�������Һ�еμ�����������Һ��������Һ��
�������£���ijŨ�ȵ�������Һ�еμ�����������Һ��������Һ��![]() ��
��![]() �仯��ͼ��ʾ������˵����ȷ����( )
�仯��ͼ��ʾ������˵����ȷ����( )
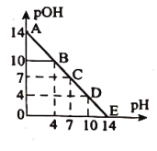
A.������![]() ��Һ��Ũ�����
��Һ��Ũ�����
B.![]() ���
���![]() ��ˮ�ĵ���̶���ͬ
��ˮ�ĵ���̶���ͬ
C.�μ�![]() ��Һ��Ϊ��ˮϡ�ͣ���ͼ���߲���
��Һ��Ϊ��ˮϡ�ͣ���ͼ���߲���
D.�����¶ȣ��ζ�������pH+pOH��14
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���õ���ʽ��ʾ���л����
(1)NaCl _____��
(2)H2 ______��
(3)MgCl2 ______��
(4)CH4 ______��
(5)CO2 ______��
(6)Na2O ______��
������ֻ�����ۼ������ʵĵ���ʽ�ijɽṹʽ�� ______�� ______�� ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪X+��Y2+��Z-��W2-�������Ӿ�������ͬ�ĵ��Ӳ�ṹ�����й���X��Y��Z��W����Ԫ�ص�����������ȷ����( )
A.ԭ��������Y��X��Z��WB.���Ӱ뾶��X+��Y2+��Z-��W2-
C.ԭ��������������Z��W��Y��XD.��ԭ�ԣ�X��Y��W2-��Z-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����õ绯ѧԭ������NO2��O2������KNO3�Ƴ�ȼ�ϵ����ģ�ҵ��ⷨ��������Cr2O72-��ˮ������ͼ��ʾ������������Һ������Ӧ��Cr2O72-��6Fe2+��14H+��2Cr3+��6Fe3+��7H2O
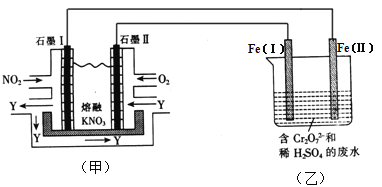
��1���׳ع���ʱ��NO2ת�����ɫ������Y��Y��N2O5����ѭ��ʹ�á���ʯī���ǵ�ص� ����ʯī�������ĵ缫��ӦʽΪ ��
��2������ʱ���׳��ڵ�NO3-������ ���ƶ����ʯī��ʯī��������ͬ�����£����ĵ�O2��NO2�������Ϊ ��
��3���ҳ���Fe(��)���Ϸ����ĵ缫��ӦΪ ��
��4������Һ�м�����0.01 mol Cr2O72-�����·������ת���� mol���ӡ�
��5������ȫ��ԭΪCr3+���ҳع�ҵ��ˮ�еμ�NaOH��Һ���ɽ�����Cr(OH)3��������ʽ��ȥ����֪Cr(OH)3���������ܽ�ƽ�⣺Cr(OH)3(s) ![]() Cr3+(aq)��3OH-(aq)��������Cr(OH)3���ܶȻ�Ksp=c(Cr3+)��c3(OH-)=1.0��10-32��Ҫʹc(Cr3+)����10��5mol��L-1����Һ��pHӦ���� ��
Cr3+(aq)��3OH-(aq)��������Cr(OH)3���ܶȻ�Ksp=c(Cr3+)��c3(OH-)=1.0��10-32��Ҫʹc(Cr3+)����10��5mol��L-1����Һ��pHӦ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ���ҳ����������������Ϊ36.5%���ܶ�Ϊ1.20 g��mL��1��
��1����Ũ������HCl�����ʵ���Ũ����________��
��2������100 mL��Ũ���ᣬ��Ҫ��״����HCl�����Ϊ________��
��3������100 mL 3.00 mol��L��1�����ᣬ������Ũ��������Ϊ________��
��4����10.0 mL 3.00 mol��L��1��������10.0 mL 1.00 mol��L��1��MgCl2��Һ��ϣ�������Һ��Cl�������ʵ���Ũ����________��(���Ϻ���Һ���������)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���״����¡��״洢��ֵ�ߡ����ܻ���������ȼ�ϣ�������Ҫ�Ļ���ԭ�ϡ���ѧ����̫���ֽܷ�ˮ���ɵ������ڴ�����������![]() ��Ӧ���ɼ״�����֪��
��Ӧ���ɼ״�����֪��![]() ��
��![]() ��
��![]() ��ȼ����H�ֱ�Ϊ
��ȼ����H�ֱ�Ϊ![]() ��
��![]() ��
��![]() ����ش��������⣺
����ش��������⣺
(1)��̫���ֽܷ�![]() ˮ���ĵ�������________
ˮ���ĵ�������________![]() ��
��
(2)�״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽΪ________��
(3)![]() ������ϳɼ״�(��Ӧ��)�а����ŷ�Ӧ��ķ�����
������ϳɼ״�(��Ӧ��)�а����ŷ�Ӧ��ķ�����
��Ӧ��![]()
��Ӧ��![]()
�ں�ѹ��![]() ��
��![]() ����ʼ��һ���������£�
����ʼ��һ���������£�![]() ƽ��ת���ʺ�ƽ��ʱ
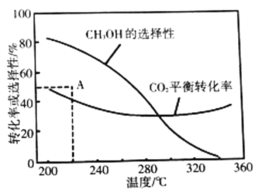
ƽ��ת���ʺ�ƽ��ʱ![]() ��ѡ�������¶ȵı仯��ͼ�����У�
��ѡ�������¶ȵı仯��ͼ�����У�![]() ��ѡ����
��ѡ����![]()
���¶ȸ���300�棬![]() ƽ��ת�������¶����߶�������ԭ����_________��
ƽ��ת�������¶����߶�������ԭ����_________��
��220��ʱ���ڴ���������![]() ��
��![]() ��Ӧһ��ʱ����
��Ӧһ��ʱ����![]() ��ѡ����Ϊ48%(ͼ��A��)�����ı䷴Ӧʱ����¶ȣ�һ�������
��ѡ����Ϊ48%(ͼ��A��)�����ı䷴Ӧʱ����¶ȣ�һ�������![]() ѡ���ԵĴ�ʩ��_________________(д������)��
ѡ���ԵĴ�ʩ��_________________(д������)��
(4)�����Ϊ![]() ���ܱ������г���
���ܱ������г���![]() ��
��![]() ������Ӧ��ƽ��ʱ������ת����Ϊ25%����÷�Ӧ��ƽ�ⳣ��K=________��(���������λ��Ч����)
������Ӧ��ƽ��ʱ������ת����Ϊ25%����÷�Ӧ��ƽ�ⳣ��K=________��(���������λ��Ч����)
(5)�о�֤ʵ��![]() Ҳ����������Һ���ö��Ե缫������ɼ״������ɼ״��ĵ缫��ӦʽΪ__________����һ����������________________��
Ҳ����������Һ���ö��Ե缫������ɼ״������ɼ״��ĵ缫��ӦʽΪ__________����һ����������________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ�����ӵ�������ֵ��N��ʾ������������������ȷ���ǣ� ��
A.6.0gSiO2�����к��й��ۼ��ĸ���Ϊ0.2NA
B.��1molCl2ͨ��ˮ�У���N��HClO����N��Cl-����N��ClO-��=2NA
C.3.0g����ȩ(HCHO)�ı������к��е�ԭ������Ϊ0.4NA
D.��CO2ͨ��Na2O2ʹ����������agʱ����Ӧת�Ƶĵ�����Ϊ![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�ϳ��л������ϵ��м��壬�ṹ��ʽ��ͼ��ʾ������˵����ȷ����
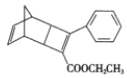
A.���ڷ�����B.����ʽΪC18H16O2
C.�����ϵĶ���ȡ������6��D.�����ȶ�������ǿ�ᡢǿ�ǿ��������Ӧ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com