

| A����MnO2������뵽FeSO4��Һ�У��ټ���KSCN��Һ���۲���Һ�Ƿ��� |
| B����MnO2������뵽FeCl3��Һ�У��ټ���KSCN��Һ���۲���Һ�Ƿ��� |
| C����MnO2������뵽Na2SO3��Һ�У��ټ���BaCl2�۲��Ƿ��а�ɫ�������� |
| D����MnO2������뵽ϡ�����У��۲��Ƿ��л���ɫ�������� |
| ʵ�� | ���� | ���� |
| A | 1��0.2mol/LNaOH��Һ | ����ɫ |
| B | 1��ˮ | ������dz�غ�ɫ |
| C | 1��0.1mol/L������Һ | Ѹ�ٱ��غ�ɫ |
 ��������
�������� ������
������ ����
����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
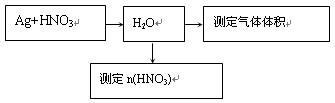
 2Fe+ Al2O3��ij�о�С����ʵ�������ü���װ�ý������ȷ�Ӧ���������ɵ��������ɵĺ�ɫӲ�顣С���Ա�Ʋ���Ҫԭ���Dz����������ʽ϶࣬����һ��̽���ʺ�ɫӲ�����ɡ�
2Fe+ Al2O3��ij�о�С����ʵ�������ü���װ�ý������ȷ�Ӧ���������ɵ��������ɵĺ�ɫӲ�顣С���Ա�Ʋ���Ҫԭ���Dz����������ʽ϶࣬����һ��̽���ʺ�ɫӲ�����ɡ�| ʵ����� | Ԥ������ͽ��� |
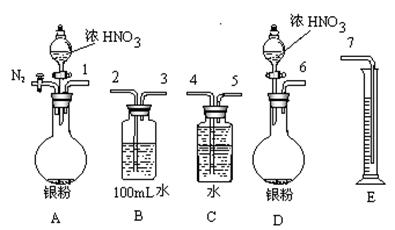
 ����1��ȡ������ĩ���ձ��У���������3mol/LNaOH��Һ����ֽ��裬���ˣ�ϴ�ӡ� ����1��ȡ������ĩ���ձ��У���������3mol/LNaOH��Һ����ֽ��裬���ˣ�ϴ�ӡ� | |
| ����2��������1������ת�Ƶ��ձ�B�У��������� ����ֽ��裬���ˣ�ϴ�ӡ� | �к�ɫ�������ɣ�˵����ĩ�к��� �� |
| ����3��������2������ת�Ƶ��ձ�C�У� �� | �� ˵����ĩ�к���Fe2O3��(1��) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
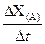 ��rX(A)��ʾ����A����������������Ũ�ȵȣ��ĸı�����ijѧϰС���ÿ�״��п��200mLϡ���ᷴӦ�о���ѧ��Ӧ���ʣ�ʵ��װ��ͼ����ͼ��
��rX(A)��ʾ����A����������������Ũ�ȵȣ��ĸı�����ijѧϰС���ÿ�״��п��200mLϡ���ᷴӦ�о���ѧ��Ӧ���ʣ�ʵ��װ��ͼ����ͼ��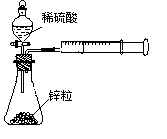
| ��� | ��Ӧ���ʱ���ʽ�Ķ��� | ��Ӧ���ʱ���ʽ | ��Ӧ���ʵ�λ |
| �� | ��λʱ����H+Ũ�ȵı仯�� | V(H+) =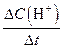 | mol / L.min |
| �� | �� | �� | �� |
| �� | ��λʱ��������H2����µ���� | V(H2) = 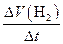 | L / min |
| ��� | п����״ | п�Ĺ�� | �����Ũ�� | ��Ӧ�¶� | ʵ����Ƶ�Ŀ�� |
| �� | ��״ | ��п | 1mol/L | 250C | ʵ��ٺ͢��о���Ŀ���� �� �� ʵ��ں͢��о�����Ũ�ȶԷ�Ӧ����Ӱ�죻 ʵ��ۺ͢��о�п�Ĺ��Է�Ӧ����Ӱ�죻 ʵ��ܺ͢��о���Ŀ���� �� �� |
| �� | ����״ | ��п | 1mol/L | 250C | |
| �� | ����״ | ��п | �� | 250C | |
| �� | ����״ | �� | 2mol/L | 250C | |
| �� | ����״ | ��п | 2mol/L | 350C | |
| ���� | |||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
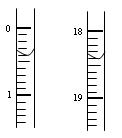
| | �ζ�ǰ������/mL | �ζ������/mL |
| ��һ�� | | |
| �ڶ��� | 0.10 | 18.00 |
| ������ | 0.20 | 18.30 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A�����Ը��������Һ | B������������Һ | C�����Ȼ�̼ | D�����軯����Һ |
| ͬѧ��ȷ | ѡ���Լ� | ʵ������ |
| ��һ�ַ��� | | |
| �ڶ��ַ��� | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
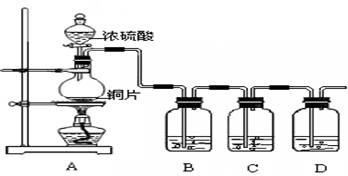
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
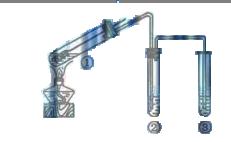
A�������ƶ�����ͭ˿�ɿ���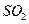 ���� ���� |
B������ѡ��Ʒ����Һ��֤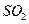 ������ ������ |
C������ѡ�ãΣ�ϣ���Һ���ն����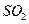 |
D��Ϊȷ�� ���ɣ�����м�ˮ���۲���ɫ ���ɣ�����м�ˮ���۲���ɫ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com