
����Ŀ��øָ������������ܵĸ߷������ʣ���ø�Ĵ���Ӧ��ϵ�У���Ӧ����ӱ���Ϊ�������ͨ��ø�Ĵ�ת��Ϊ�������ӣ��������е�ϸ������̶���Ҫø�IJ��룬�����Ч�ʣ���ش��������⣺
��1��ø����ø����������ϸ������ʵ�ַ����ţ�����Ϊϸ���ھ��� ϵͳ����ɸ�ϵͳ�Ľṹ���еĹ��������� ����Ҷϸ����Ҳ�����ڶ�����ķ��������������ø���̲���ȡ�����б����Ƚ����ȹ����³��ƣ���һ���̵�Ŀ���� ��
��2��������Rubiconø��CO2Ũ�Ƚϸ�ʱ����ø��C2��CO2��Ӧ��Rubiconø�Ĵ��ڳ���Ϊ ����ø���С������ԡ�����O2Ũ�Ƚϸ�ʱ����ø��C2��O2��Ӧ�����ᆳһϵ�б仯���������л����CO2���䡰�����ԡ���ø�� �����ԣ���ì�ܣ�
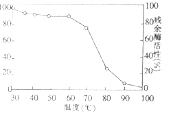
��3����ͼ�����߱�ʾ��ø�ڲ�ͬ�¶��±����㹻��ʱ�䣬����ø������ߵ��¶��²������ø���ԣ���ͼ�ɵó��� ��Ϊ����֤��һ���ۣ��������µ���ά�طֽ�øΪʵ����ϣ��Ƚ��ڵ��º������¶��´����ø���Ե�Ӱ�죬д��ʵ�����˼·�� ��
���𰸡���1������Ĥ ѡ������ ����ʹ������øʧ��
��2��Ҷ������� רһ��
��3��ø�ڽϵ��¶��´��������Ӱ��С���ڽϸ��¶��´��������Ӱ���
�����µ���ά�طֽ�ø�ֱ��ڵ��º������¶��´����㹻��ʱ������������¶��²��������
��������
�����������1��ø����ø����������ϸ������ʵ�ַ����ţ�����Ϊϸ���ھ�������Ĥϵͳ������Ĥ�Ľṹ�Ĺ���������ѡ�����ԣ��̲���ȡ�����б����Ƚ����ȹ����³��ƣ���һ���̵�Ŀ���Ǹ���ʹ������øʧ�
��2��������Rubiconø��CO2Ũ�Ƚϸ�ʱ����ø��C2��CO2��Ӧ���÷�Ӧ���ڰ���Ӧ�е����ʱ仯�����Rubiconø�Ĵ��ڳ���ΪҶ������ʣ���ø���С������ԡ�����O2Ũ�Ƚϸ�ʱ����ø��C2��O2��Ӧ�����ᆳһϵ�б仯���������л����CO2���䡰�����ԡ���ø��רһ����ì�ܣ�
��3����ͼ�����߱�ʾ��ø�ڲ�ͬ�¶��±����㹻��ʱ�䣬����ø������ߵ��¶��²������ø���ԣ���ͼ�ɵó�ø�ڽϵ��¶��´��������Ӱ��С���ڽϸ��¶��´��������Ӱ���Ϊ����֤��һ���ۣ����Խ����µ���ά�طֽ�ø�ֱ��ڵ��º������¶��´����㹻��ʱ������������¶��²�������ԣ�
�ʴ�Ϊ����1������Ĥ ѡ������ ����ʹ������øʧ��
��2��Ҷ������� רһ��
��3��ø�ڽϵ��¶��´��������Ӱ��С���ڽϸ��¶��´��������Ӱ���
�����µ���ά�طֽ�ø�ֱ��ڵ��º������¶��´����㹻��ʱ������������¶��²��������


 ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij������������Fe2O3��Ϊ�缫�����Ʊ�����ӵ�أ���һ��Ϊ����﮺�ʯī�ĸ��ϲ��ϣ���ͨ�������������¶�����ӵ�ؽ���ѭ����ŵ磬�ɹ���ʵ���˶Խ��ԵĿ�����أ���ͼ������˵����ȷ���ǣ� ��

A. �õ�ؿ�����NaOH��ҺΪ�������Һ
B. �ŵ�ʱ��������ĵ缫��ӦʽΪFe2O3+6Li++6e-�T3Li2O+2Fe
C. ���ʱ��Fe��Ϊ��������ز�����������
D. ��������Ҫ�ɷ���Fe3O4����ȿ���������Ҳ������Fe2O3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪1molSiO2����4mol Si��O���йؼ�������������������������ȼ�յ��Ȼ�ѧ����ʽ��Si(s)��O2(g)��SiO2(s)����H����989.2kJ��mol��1�������x��ֵΪ
��ѧ�� | Si��O | O��O | Si��Si |
����/kJ��mol��1 | x | 498.8 | 176 |
A. 460 B. 920 C. 1165.2 D. 423.3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������(POCl3)���������ȡ����Ȼ��ף�����Ҫ�Ļ�������ԭ�ϣ���������ȡȾ�ϡ�ũҩ���л��ϳɵ��Ȼ�������������ȼ���ȡ�ij��ѧʵ��С��ģ��PCl3ֱ���������Ʊ�POCl3,��ʵ��װ��������£�
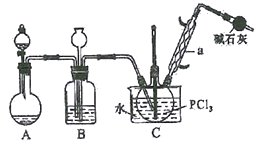
�й����ʵIJ����������±���
�۵�/�� | �е�/�� | ���� | |
PCl3 | -112 | 75.5 | ��ˮ����H3PO3��HCl����O2����POCl3 |
POCl3 | 2 | 105.3 | ��ˮ����H3PO4��HCl��������PCl3 |
�ش��������⣺
��1������a��������__________��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ__________________��
��2��B����װ�Լ�Ϊ__________��Bװ�õ����ó��۲�O2������֮�⣬����_____________________��
��3��Cװ�ÿ��Ʒ�Ӧ��60��~65�����У�����µ���ҪĿ����__________��
��4��ʵ���Ƶõ�POCl3�ֲ�Ʒ�г�����PCl3������__________�����ᴿ��
��5��ͨ��������·����Բⶨ�������ײ�Ʒ��ClԪ�غ�����ʵ�鲽�����£�
����ȡag��Ʒ����ƿ�У���������NaOH��Һ������ȫ��Ӧ���ϡ���������ԡ�
��������ƿ�м���0.1000mol��L-1��AgNO3��Һ40.00mL,ʹCl-��ȫ������
���������м���2mL������������ҡ����ʹ�������汻�л��︲�ǣ��Է�ֹ�ڵμ�NH4SCNʱ����AgCl����ת��ΪAgSCN������
��������ָʾ������cmol��L-1NH4SCN��Һ�ζ�����Ag+���յ㣬�����������VmL��
��֪��Ksp(AgCl)=3.2��10-10,Ksp(AgSCN)=2��10-12
�ٵζ�ѡ�õ�ָʾ����__________ (����)��
a��FeCl2 b������ c������ d��NH4Fe(SO4)2
��ClԪ�ص������ٷֺ���Ϊ__________ (�г���ʽ)��
���ڲ������У��������������IJ���������Cl-Ԫ�غ�������__________(����ƫ������ƫС������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ԫ��A��B��C��D,ԭ��������������A�Ļ�̬ԭ�ӵ�L�������K����ӵ�������B�ļ۵��Ӳ��е�δ�ɶԵ�����3����C��Bͬ����D����ۺ�����Ϊ������ǿ���������ᡣ��ش��������⣺
��1��C�Ļ�̬ԭ�ӵĵ����Ų�ʽΪ___________��D����ۺ��������Ա�������۵ĺ���������ǿ��ԭ����_____________________________________________��
��2���ӻ������Ϊ���ԺͲ������ӻ����������ӻ�ʱ���ӻ�������в��μӳɼ��ŵ��ӶԵĴ��ڡ�A��B��C���ֱ�����D�γ�����ԭ���ӻ���ʽ��Ϊ___________�Ĺ��ۻ�����X��Y��Z�����У����ڲ������ӻ�����___________ (д��ѧʽ)��
��3���Ƚ�Y��Z���۷е�Y______Z(����>������<������=��)������������_____________________��
��4��DԪ������Cu�γ��ػ�ɫ���壬��ˮ�ܽⲢϡ�����У���Һ��ɫ����ɫ��ת��Ϊ��ɫ������ɫ������_________________������ɫ�����е���λԭ��________________��
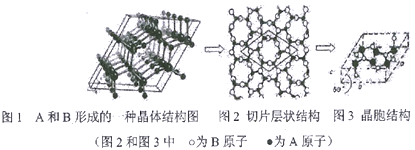
��5��A��B���γɶ��ֽṹ�ľ��塣����һ������ʯī�Ľṹ����ṹ����ͼ��ʾ(ͼ1Ϊ����ṹ��ͼ2Ϊ��Ƭ��״�ṹ)���仯ѧʽΪ__________��ʵ���ô˾���ṹ����������ϵ,�����ṹ��ͼ3����֪ͼʾԭ�Ӷ������ھ����ڣ���������a=0.64nm��c=0.24nm���侧���ܶ�Ϊ__________g/cm3(��֪��2=1.414,��=1.732,�����ȷ��С������2λ��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±������ʵķ��������ȫ��ȷ����
��� | A | B | C | D |
ǿ����� | KNO3 | H2SO4 | BaSO4 | HClO4 |
������� | HClO4 | CaCO3 | HClO | NH3��H2O |
�ǵ���� | SO2 | CS2 | H2O | C2H5OH |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��һ����ѧ���̵�ʾ��ͼ:
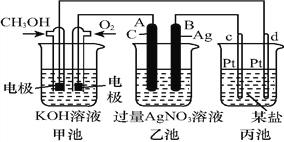
��ش�:
��1��ͼ�м׳���______װ�ã�����OH������_________��(����CH3OH������O2��)��
��2��д��ͨ��CH3OH�缫�ĵ缫��Ӧʽ:_______________________________��
��3�����ҳ����缫�����μ�������ɫʯ����Һ���������ĵ缫Ϊ_____��(����A������B��)��
��д���˵缫�ĵ缫��Ӧʽ:______________________��
��4���ҳ��з�Ӧ�����ӷ���ʽΪ________________________��
��5�����ҳ���B(Ag)����������5.40 gʱ���ҳ�c(H+)��_______(���ҳ�����ҺΪ500 mL)����ʱ����ij�缫����1.60 gij����������е�ij����Һ������_________(�����)��
A��MgSO4 B��CuSO4 C��NaCl D��AgNO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�pH�仯���ж��У���ȷ������ ��
A. ���¶ȵ����ߣ���ˮ��Kw��С
B. ���¶ȵ����ߣ���ˮ��pH����
C. ������ˮ������һ��ʱ���pH��С
D. ����������Һ�����ڿ����У�pH����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������0.1000 mol��L��1KMnO4������Һ�ζ�δ֪Ũ�ȵ���ɫH2C2O4��Һ����Ӧ���ӷ���ʽ�ǣ�2MnO4����5H2C2O4��6H+ = 2Mn2+��10CO2����8H2O
���������⣺
��1���õζ�ʵ������IJ���������______________��������ĸ��
A����ʽ�ζ���B����ʽ�ζ��� C����Ͳ D����ƿ E������̨F���ζ��ܼ�G���ձ�H����ֽ I��©��
��2������________(��ᡱ�)ʽ�ζ���ʢ�Ÿ��������Һ���Է���ԭ��___________________________________________��
��3���ζ��յ������Ϊ___________________________________��
��4�����ζ���ʼ�ͽ���ʱ���ζ����е�Һ����ͼ��ʾ������ʼ����Ϊ________mL���յ����Ϊ________mL��
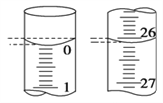
��5��ijѧ������3��ʵ��ֱ��¼�й��������±���
�ζ� ���� | ����H2C2O4��Һ�����/mL | 0.1000 mol/L KMnO4�������mL�� | ||
�ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
�ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
������ | 25.00 | 0.22 | 26.31 | 26.09 |
�����ϱ�������ʽ�����H2C2O4��Һ�����ʵ���Ũ��Ϊ_______________��
��6�����в����п���ʹ�ⶨ���ƫ�͵���___________(����ĸ)��
A����ʽ�ζ���δ�ñ�Һ��ϴ��ֱ��ע��KMnO4��Һ
B���ζ�ǰʢ�Ų�����Һ����ƿ������ˮϴ����û�и���
C����ʽ�ζ��ܼ��첿���ڵζ�ǰû�����ݣ��ζ���������
D����ȡKMnO4��Һʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com