
����Ŀ������0.1000 mol��L��1KMnO4������Һ�ζ�δ֪Ũ�ȵ���ɫH2C2O4��Һ����Ӧ���ӷ���ʽ�ǣ�2MnO4����5H2C2O4��6H+ = 2Mn2+��10CO2����8H2O
���������⣺
��1���õζ�ʵ������IJ���������______________��������ĸ��
A����ʽ�ζ���B����ʽ�ζ��� C����Ͳ D����ƿ E������̨F���ζ��ܼ�G���ձ�H����ֽ I��©��
��2������________(��ᡱ�)ʽ�ζ���ʢ�Ÿ��������Һ���Է���ԭ��___________________________________________��
��3���ζ��յ������Ϊ___________________________________��
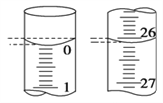
��4�����ζ���ʼ�ͽ���ʱ���ζ����е�Һ����ͼ��ʾ������ʼ����Ϊ________mL���յ����Ϊ________mL��
��5��ijѧ������3��ʵ��ֱ��¼�й��������±���
�ζ� ���� | ����H2C2O4��Һ�����/mL | 0.1000 mol/L KMnO4�������mL�� | ||
�ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
�ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
������ | 25.00 | 0.22 | 26.31 | 26.09 |
�����ϱ�������ʽ�����H2C2O4��Һ�����ʵ���Ũ��Ϊ_______________��
��6�����в����п���ʹ�ⶨ���ƫ�͵���___________(����ĸ)��
A����ʽ�ζ���δ�ñ�Һ��ϴ��ֱ��ע��KMnO4��Һ
B���ζ�ǰʢ�Ų�����Һ����ƿ������ˮϴ����û�и���
C����ʽ�ζ��ܼ��첿���ڵζ�ǰû�����ݣ��ζ���������
D����ȡKMnO4��Һʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
���𰸡� ADG �� ������ؾ���ǿ�������ܸ�ʴ�ܣ�����������Һ�ܰ��������� �������һ�θ��������Һʱ����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ��ָ�ԭɫ 0.00 26.10 0.2610 mol/L CD
����������1����������ԭ�ζ����貣�������У�ʢװ����KMnO4��Һ��ȡ�ô���ҺH2C2O4��Һ����ʽ�ζ��ܡ�ʢװ����ҺH2C2O4��Һ����ƿ������Һ�漰������ʱʢ����Һ�õ��ձ�������ӦѡADG��
��2����Ϊ������ؾ���ǿ�������ܸ�ʴ�ܣ����Բ��ü�ʽ�ζ���ʢ�Ÿ��������Һ��
��3��MnO4-Ϊ��ɫ��K+Ϊ��ɫ�����������һ�θ��������Һʱ����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ��ָ�ԭɫ����˵���ﵽ�ζ��յ㡣
��4����ͼ��ʾ������ʱ����Ӧ�밼Һ����ʹ����У�����ʼ����Ϊ0.00mL���յ����Ϊ26.10mL��
��5����2������������������ϴ������ݵ���Ч�ԣ�Ӧ��ȥ��2�����ݣ����ĵ�V(KMnO4)=(26.11+26.09)mL��2=26.10mL���ɷ���ʽ2MnO4����5H2C2O4��6H+ = 2Mn2+��10CO2����8H2O�ɵ���n(H2C2O4)= ![]() n(MnO4-)������c(H2C2O4)��0.025L=
n(MnO4-)������c(H2C2O4)��0.025L=![]() ��0.1000molL-1��0.02610L�����c(H2C2O4)=0.2610molL-1��
��0.1000molL-1��0.02610L�����c(H2C2O4)=0.2610molL-1��
��6������c(��)= 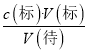 ������������A���ʽ�ζ���δ�ñ�Һ��ϴ��ֱ��ע��KMnO4��Һ�����Һ��Ũ��ƫС�����V(��)ƫ��c(��)ƫ��A����B��ζ�ǰʢ��H2C2O4��Һ����ƿ������ˮϴ����û�и����V(��)û��Ӱ�죬c(��)���䣬��B����C���ʽ�ζ��ܼ��첿���ڵζ�ǰû�����ݣ��ζ��������ݣ����V(��)ƫС��c(��)ƫС����C��ȷ��D���ȡKMnO4��Һʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ��������V(��)ƫС��c(��)ƫС����D��ȷ��
������������A���ʽ�ζ���δ�ñ�Һ��ϴ��ֱ��ע��KMnO4��Һ�����Һ��Ũ��ƫС�����V(��)ƫ��c(��)ƫ��A����B��ζ�ǰʢ��H2C2O4��Һ����ƿ������ˮϴ����û�и����V(��)û��Ӱ�죬c(��)���䣬��B����C���ʽ�ζ��ܼ��첿���ڵζ�ǰû�����ݣ��ζ��������ݣ����V(��)ƫС��c(��)ƫС����C��ȷ��D���ȡKMnO4��Һʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ��������V(��)ƫС��c(��)ƫС����D��ȷ��


 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��øָ������������ܵĸ߷������ʣ���ø�Ĵ���Ӧ��ϵ�У���Ӧ����ӱ���Ϊ�������ͨ��ø�Ĵ�ת��Ϊ�������ӣ��������е�ϸ������̶���Ҫø�IJ��룬�����Ч�ʣ���ش��������⣺
��1��ø����ø����������ϸ������ʵ�ַ����ţ�����Ϊϸ���ھ��� ϵͳ����ɸ�ϵͳ�Ľṹ���еĹ��������� ����Ҷϸ����Ҳ�����ڶ�����ķ��������������ø���̲���ȡ�����б����Ƚ����ȹ����³��ƣ���һ���̵�Ŀ���� ��
��2��������Rubiconø��CO2Ũ�Ƚϸ�ʱ����ø��C2��CO2��Ӧ��Rubiconø�Ĵ��ڳ���Ϊ ����ø���С������ԡ�����O2Ũ�Ƚϸ�ʱ����ø��C2��O2��Ӧ�����ᆳһϵ�б仯���������л����CO2���䡰�����ԡ���ø�� �����ԣ���ì�ܣ�
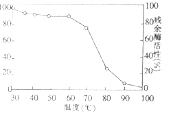
��3����ͼ�����߱�ʾ��ø�ڲ�ͬ�¶��±����㹻��ʱ�䣬����ø������ߵ��¶��²������ø���ԣ���ͼ�ɵó��� ��Ϊ����֤��һ���ۣ��������µ���ά�طֽ�øΪʵ����ϣ��Ƚ��ڵ��º������¶��´����ø���Ե�Ӱ�죬д��ʵ�����˼·�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������Ԫ��֮һ��̼���䵥�ʼ����������о�����������������Ҫ��;����ش���������:
��1����̬̼ԭ�Ӽ۵���ԭ�ӹ����ʾʽΪ________���������________�ֿռ��˶�״̬�ĵ���.
��2�� �л����ж�����̼ԭ�ӣ����̼ԭ�ӵ����Ƶijɼ��ص��йأ��Խ����л���������ԭ��________ ��
��3�� �Ƚ�����̼������һ���¶��»ᷢ���ֽ���¶ȺͶ�Ӧ�������Ӱ뾶��������仯���ɼ�ԭ��_____________��
̼���� | MgCO3 | CaCO3 | BaCO3 | SrCO3 |
�ȷֽ��¶�/�� | 402 | 900 | 1172 | 1360 |
�����˰뾶/pm | 66 | 99 | 112 | 135 |
��4��1828 �꣬�¹���ѧ������(F��Wohler)������������ѧ˵����������ʵ�����ォ���������(NH4CNO)��Һ�������õ����л�������[CO(NH2)2]��������C��N��O��һ�����ܴ�С˳��Ϊ____________��
��5�� ̼��ͬ���������ж��֣�����һ��Ϊʯī��ϵƽ���״�ṹ��ͬһ����ÿ��̼ԭ������������̼ԭ����C -C ����������ƽ���������Σ��Ҳ����Ի��������Ľṹ��ͼ��ʾ������̼ԭ�ӵ��ӻ���ʽΪ_______�������ڵ�������Ϊ__________��
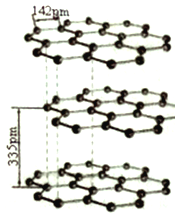
��6��ʯī�����ά�ṹ�������ԡ�
��ͬһ�����ڵ�ƽ����Ԫ�����ṹ(����ͼ)���ں�lmolCԭ�ӵ�ʯī�У���_____��ƽ���������Ρ�
����֪ʯī����Ϊ335pm��C-C����Ϊ142pm�����ܶ�______g/cm3(��֪��lpm=10-10cm���г�����ʽ����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͨ����70������ڷ�չ������һ������ͨ�ż�����Ŀǰ���������ͨ��ϵͳ��Ͷ��ʹ�ã�����ͨ�ŵĹ��ά���������������ʾ�������Ƴɵģ� ��
A.ʯī
B.ʯӢ
C.ʯ��ʯ
D.�ߴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ȤС���ͬѧ����ͼ��ʾװ���о��йص绯ѧ������(�ס��ҡ��������е���������)�����պϸ�װ�õĵ��Kʱ���۲쵽��������ָ�뷢����ƫת��

��ش���������:
(1)�׳�Ϊ_________(�ԭ��ء��������ء���Ƴء�)��A�缫�ĵ缫��ӦʽΪ______��
(2)������F�缫Ϊ____(�����������������������������������)���ó��ܷ�Ӧ�ķ���ʽΪ_____��
(3)���ҳ���C����������4.32gʱ���׳���B�缫����������O2�����Ϊ_____mL(��״��)��
(4)һ��ʱ��Ͽ����K������������ʹ�ҳػָ�����ӦǰŨ�ȵ���______(����ĸ)��
A.Cu B.CuO C.Cu(OH)2 D.Cu(OH)2CO3
(5)�״����Ҵ���������ȼ�ϡ���֪�Ҵ�Һ����ȫȼ������CO2�����1molҺ̬ˮʱ�ų�����453.3kJ����д���Ҵ�ȼ�յ��Ȼ�ѧ����ʽ:____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ĸ�����С��Ϊ���ʵ��Ŀ�ģ������������ʵ��װ�ã�����ͼ�еļг�װ����ȥ��������һ���С����Ƶ�����װ���У���һ��װ�ô���ԭ�����ûС����
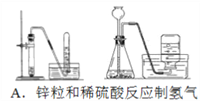
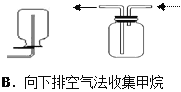
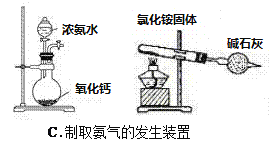
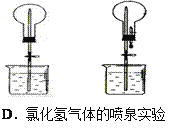
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ������������أ�����Ӧ�������������������Ե��ǣ� ��
A.��������ˮ
B.�ú�ϡ�ĸ��������Һ����
C.ʳ�׳�ˮ��
D.��������Ư�ײ�ñ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ�ⶨij���ᾧ����H2C2O42H2O����Ʒ�Ĵ������ֳ�ȡһ�������ĸ���Ʒ�����Ƴ�100 mL��Һ��ȡ25.00 mL����Һ����ƿ����������ϡ��������0.100 mol/L��KMnO4��Һ�ζ�(���ʲ����뷴Ӧ)��Ϊʡȥ������̣�����ȡ����Ʒ������Ϊij��ֵʱ���ζ�����KMnO4��Һ�ĺ�����ǡ�õ�����Ʒ�в��ᾧ�������������100������Ӧ��ȡ��Ʒ������Ϊ
A. 2.25 g B. 3.15 g C. 9.00 g D. 12.6 g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Na2CO3��Һ��ȡNaHCO3��Һ��NaOH��Һ��װ������ͼ��ʾ������˵���У�����ȷ����
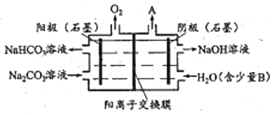
A. ��������������A��H2
B. ��Һ��Na+����������������Ǩ��
C. ����B��NaCl������������ǿ��Һ������
D. ����OH-�ŵ磬H+Ũ������CO32-ת��ΪHCO3-
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com