
�輰�仯����������ִ�������������ף���ش������й����⣺
(1)��ԭ�ӵĽṹʾ��ͼ��________��
(2)������Ʒ���豸���õIJ������ڹ����ε���________��
�ٳ�����Ͽˮ���ӣ���ʯӢ���ά�����մ�����������ͨ�������ݹ�̫���ܵ��
A���٢ڢ� B���ۢܢ� C���ڢۢ� D���٢ۢ�
(3)�����£�SiCl4ΪҺ̬���е�Ϊ57.6�棬�ڿ�����ð�������Ʊ��ߴ��ȹ���м����SiCl4������Һ̬���ʣ���Ҫ�õ��ߴ���SiCl4��Ӧ���õķ�����________���û�ѧ����ʽ����Ҫ���ֽ���SiCl4�ڿ�����ð������ԭ��_______________________________________��
(4)��ҵ�Ͽ���SiCl4(g)�Ʊ����½ṹ�մɵ����裬�䷴Ӧ����ʽΪ
3SiCl4(g)��2N2(g)��6H2(g) Si3N4(s)��12HCl(g)����H��a kJ/mol(a��0)
Si3N4(s)��12HCl(g)����H��a kJ/mol(a��0)
�ٸ÷�Ӧ��ƽ�ⳣ������ʽK��______________.
�����ܱպ��������У��ܱ�ʾ������Ӧ�ﵽƽ��״̬����________��
A��3v��(N2)��v��(H2)
B��v��(HCl��4v����4v��(SiCl4)
C����������ܶȱ��ֲ���
D��c(N2)��c(H2)��c(HCl)��1��3��6
����ij�����´ﵽƽ��ʱ��H2��HCl���ʵ���֮��Ϊm��n�����������������䣬�����¶ȴﵽƽ��ʱ��H2��HCl���ʵ���֮��________m��n(�>��������������)��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��γ�ȥ���и���ĩ״������е�����(������Ϊ����)����ѡ�������ṩ���Լ��Ͳ�������������ڱ��ڡ�
��ѡ�Լ���A���B�ռ���Һ��C������Dˮ��ECO2��F���������Լ�
��ѡ�������ټ��ȣ��ڼ������ڣ��۹��ˣ��ܽᾧ
| �����ʵ����� | �����Լ� | ��Ҫ���� |
| (1)SiO2(NaHCO3) | | |
| (2)SiO2(CaCO3) | | |
| (3)SiO2(Si) | | |
| (4)NaCl(SiO2) | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��A��J�ֱ������ط�Ӧ��һ�����ʡ���֪A�ֽ�õ������ʵ�����B��C��D��ͼ���в���������δ�����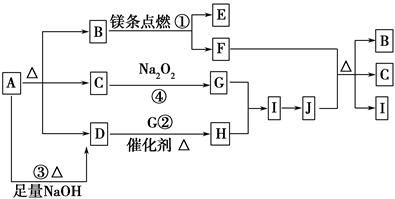
��֪��2Mg��CO2 2MgO��C������д���¿հף�
2MgO��C������д���¿հף�
(1)A�Ļ�ѧʽ________��
(2)д����Ӧ�ڵĻ�ѧ����ʽ��________________________________________��
(3)д����Ӧ�۵����ӷ���ʽ��________________________________________��
(4)J��F��Ӧ�Ļ�ѧ����ʽ��_________________________________________��
(5)�ڷ�Ӧ���У������ɱ�״����2.24 L Gʱ��ת�Ƶ�����Ϊ________ mol��
�鿴�𰸺ͽ���>>
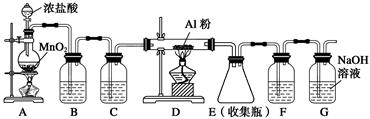
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)�����뺬�Ȼ������йص�˵����ȷ����________(����ĸ)��
| A��HClO�����ᣬ����NaClO��������� |
| B�����ˮ����μ�����������FeCl3��Һ�����Ƶ�Fe(OH)3���� |
| C��HCl��Һ��NaCl��Һ��ͨ�����ӵ��磬����HCl��NaCl�������ӻ����� |
| D�����NaCl��Һ�õ�22.4 L H2(��״��)����������Ҫת��NA������(NA��ʾ�����ӵ�����) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ�١���ֱ�����йط�Ӧ�е�һ�����ʣ����Т�������ʹ��̪��Һ��죬���Ǻ���ɫ���壬�ش�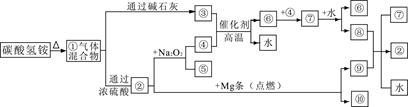
��1�����еĻ������ͨ��Ũ���ᷢ����ѧ��Ӧ����Ҫ������Ļ�ѧʽ�� ��
��2��д��̼������������ӵļ��鷽�� ��
��3��д����ҵ�Ϻϳɢ۵Ļ�ѧ����ʽ ��
��4������ᷴӦ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Na2S2O3��5H2O �׳ơ�������,�dz��õ�����������Ӱ���ͻ�ԭ��;������ɫ������ˮ�ľ���,�������Ҵ�,��20 �� ��70 �� ʱ���ܽ�ȷֱ�Ϊ60.0 g��212 g,Na2S2O3��5H2O��40��45 ���ۻ�,48 ��ֽ⡣������ʵ�����Ʊ����������ʵ�顣
�Ʊ������ķ�Ӧԭ��:Na2SO3+S Na2S2O3
Na2S2O3
�Ʊ�����������:
(1)ʵ�鿪ʼʱ��1 mL�Ҵ���ʪ��۵�����������������
A.������������������ǵij�ֽӴ�
B.��ֹ���������ܽ�
C.������Һ��pH
D.��߲�Ʒ�Ĵ���
(2)���ȹ��˵�ԭ���� ����
(3)��Һ������ֱ�������ᾧ�Ŀ���ԭ���� ����
(4)���˹�������Ҫϴ�Ӳ�Ʒ����,����Һ�����ʺϵ�������������
A.��ˮ�Ҵ� B.����NaCl��Һ C.ˮ D.��Һ
(5)��Ʒ�Ĵ��Ȳⶨ:ȡ���ò�Ʒ10.0 g,���500 mL��Һ,�ٴ���ȡ��25 mL��Һ����ƿ��,�μӼ��ε�����ָʾ��,Ȼ����0.050 mol/L�ı���ˮ��Һ�ζ�,�ظ�����,ƽ������20 mL����ˮ,�漰�ĵζ���Ӧ����ʽΪ:I2+2Na2S2O3 2NaI+Na2S4O6����Ʒ�е�Na2S2O3��5H2O�Ĵ���Ϊ��������%��
2NaI+Na2S4O6����Ʒ�е�Na2S2O3��5H2O�Ĵ���Ϊ��������%��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ƣ�NaClO2����һ��ǿ������Ư�����㷺���ڷ�֯��ӡȾ��ʳƷ��ҵ�����ڼ��Ի������ȶ����ڡ�ijͬѧ�������Ϻ��������NaClO2����Ҫ�������¡�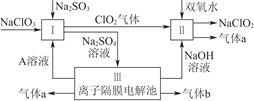
��1��˫��ˮ�ĽṹʽΪ____________�����з�����Ӧ�Ļ�ԭ����__________���ѧʽ����
��2�����з�Ӧ�����ӷ���ʽ��_____________________________________________________________________________________________________________________________________��
��3��A�Ļ�ѧʽ��________��װ�â���A��________����������
��4��ClO2��һ�ָ�Чˮ�������������������ƺ�ϡ����Ϊԭ���Ʊ���
��д���÷�Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________________________________________________________________________________��
���о�����������Ӧ��ʼʱ����Ũ�Ƚϴ��������������Cl2�������ӷ���ʽ���Ͳ���Cl2��ԭ��________________________________________________________________________
________________________________________________________________________��
��5��NaClO2���ʿɷֽ�ΪNaClO3��NaCl��ȡ������NaClO2������һ�ݸ����ʵ�����ʹ֮���ʣ���һ���ϸ棬�������Һ�����ֱ�������FeSO4��Һ��Ӧʱ������Fe2�������ʵ���________�����ͬ��������ͬ�������жϡ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���꣬Ϊ����������ʣ�����������������Ϊ��߾���Ч�棬��������������ܶ�ܻ����������̽�����ٽ���ѧ��ҵ�ķ�չ��
��1�����ڹ������̵�Ӧ�ã����÷��糧������SO2�Ƴ��Է���أ����ط�Ӧ����ʽΪ��2SO2��O2��2H2O��2H2SO4���õ�ص綯��Ϊ1.06V��ʵ�ʹ����У���SO2ͨ���ص� �����������������������ӦʽΪ �������ַ�������SO2�������ŵ��� ��
��2�������Ṥҵ��SO2β������ˮ��ʯ��ʯ����̿��̼����狀��Ȼ��ص�Ϊԭ�ϣ����Ժϳ�����ҪӦ�ü�ֵ���ơ�����ء�������淋����ʣ��ϳ�·�����£�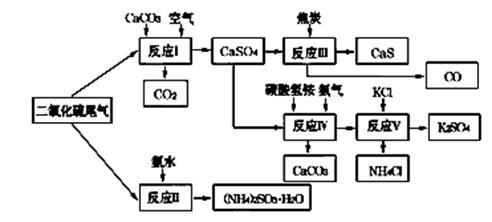
�������У���Ӧ���е���Һ�м���������ԭ�Ժ�ǿ�ĶԱ����ӵ����ʣ���Ŀ���� ��
�������й�˵����ȷ���� (�����)��
| A����Ӧ��������������������Ա�֤����������������������� |
B����Ӧ���з�����Ӧ�Ļ�ѧ����ʽΪCaSO4��4C  CaS��4CO�� CaS��4CO�� |
| C����Ӧ���������60��70�棬Ŀ��֮һ�Ǽ���̼����淋ķֽ� |
| D����Ӧ���еĸ������Ȼ�刺��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Na2SO3�dz��õĿ�������
��1��ʵ����ͨ����Ũ���ᣨ1��1����Na2SO3���Ʊ�SO2���壬
��Ӧ����ʽΪ�� ���Ʊ���SO2������ͨ������ˮ���������и�����ܸ���SO2������ǣ� ��
A.Ũ���� B.��ʯ�� C.��ˮCaCl2
��2�� ����SO2����ͨ��NaOH��Һ�пɵ�NaOH��Na2SO3�Ļ����Һ����û����Һ�м���������ˮ������Һ��Ϊ��ɫ��������Һ��Br2��Na2SO3����������ԭ��Ӧ����Ӧ�����ӷ���ʽΪ______________��
��3����Ӧ�����Һ����SO32����SO42����Br����OH���������ӣ�����д��������SO32����SO42����Br����ʵ�鱨�棻
��ѡ�Լ���2 mol��L��1HCl��1 mol��L��1H2SO4��1mol��L��1HNO3��1 mol��L��1BaCl2��
1 mol��L��1Ba(NO3)2��0.1 mol��L��1AgNO3��CCl4���������Ʊ�����ˮ�����Ʊ�����ˮ��
| ��� | ʵ����� | Ԥ������ͽ��� |
| ����� | ȡ��������Һ���Թ�A�У��μ�2 mol��L��1HCl����Һ�����ԣ����뼸��________(���Լ�)���� | ________��֤������Һ�к�SO32- |
| ����� | ��ȡ��������Һ���Թ�B�У����� ���ٵμ����� 1 mol��L��1 BaCl2��Һ | |
| ����� | ��ȡ��������Һ���Թ�C�У� �������ú�۲���ɫ | ��Һ�ֲ㣬�ϲ�Һ��ʳȺ�ɫ��֤������Һ�к�Br- |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com