
����Ŀ���Ȼ�������(�ṹ��ʽΪ )��һ�ֶ���;���л��ϳ��Լ�����HClO4-NaClO4�����У� K5[Co3+O4W12O36](��дΪCo3+W)�ɴ��ϳ��Ȼ���������
)��һ�ֶ���;���л��ϳ��Լ�����HClO4-NaClO4�����У� K5[Co3+O4W12O36](��дΪCo3+W)�ɴ��ϳ��Ȼ���������
��1����̬��ԭ�ӵĺ�������Ų�ʽΪ________________�����HClO4-NaClO4��4��Ԫ�صĵ縺����С�����˳��Ϊ____________________��
��2���Ȼ���������������ԭ�Ӻ�̼ԭ�ӵ��ӻ�������ͷֱ���____________��___________�� 1���Ȼ������������к�����������ĿΪ______________���Ȼ���������5��Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ_________________��
��3��ClO4-�Ŀռ乹��Ϊ__________________��
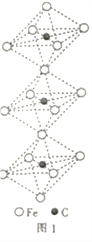
��4��һ��������̼�γɵļ�϶������ľ���ṹ��ͼ1��ʾ������̼ԭ��λ����ԭ���γɵİ���������ģ�ÿ����ԭ����Ϊ���������干�ã���û�����Ļ�ѧʽΪ________________��
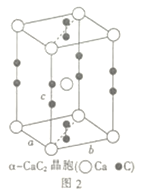
��5����ʯ(CaC2)���Ʊ��Ȼ���(ClCN)����Ҫԭ�ϡ��ķ���̼����(CaC2)�ľ����ṹ����ͼ2��ʾ���侧�������ֱ�Ϊa��b��c����a=b��c=640pm����֪�ķ���̼���Ƶ��ܶ�Ϊ1.85g��cm-3��[C��C]2-�м���Ϊ120pm����ɼ���̼ԭ�����ԭ�ӵľ���Ϊ________pm��_______pm��(�谢���ӵ���������ֵΪ6��1023)
���𰸡� 1s22s22p63s23p63d7{��[Ar]3d7} Na<H<Cl<O sp3 sp 5 N>O>Cl>C>S �������� Fe3C 260 306
����������1��1s22s22p63s23p63d7{��[Ar]3d7}��Na<H<Cl<O
��2�����Ȼ��������Ľṹ��ʽ���Կ�������Sԭ���γ���4��������û�йµ��Ӷԣ�����Sԭ�Ӳ�ȡ��sp3�ӻ���Cԭ���γ���һ��������һ����������������������û�йµ��Ӷԣ�����Cԭ����sp�ӻ���1���Ȼ������������к�����������ĿΪ5�������а���������Ԫ�صĵ�һ�����ܴ�С˳��Ϊ��N>O>Cl>C>S���ʴ�Ϊ��sp3��sp��5��N>O>Cl>C>S��
��3��ClO4-����ԭ��Cl����㹲��8�����ӣ���4��O�γ���4��������VSEPRģ��Ϊ���������ͣ�û�йµ��Ӷԣ�����ClO4-�Ŀռ乹��Ϊ���������͡�
��4��ÿ���������а�����һ��Cԭ�ӣ�һ��6��Feÿ��Fe�����������幫�ã����Ի�ѧʽΪ��Fe3C��
��5������̼ԭ�����ԭ�ӵľ���![]() ��һ�������а�����Ca��1+8��1/8=2��C2��2��1/2+4��1/4=2��һ�������а�����2��CaC2������һ������������Ϊ��
��һ�������а�����Ca��1+8��1/8=2��C2��2��1/2+4��1/4=2��һ�������а�����2��CaC2������һ������������Ϊ��![]() ������Ϊ
������Ϊ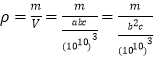 �������
�������![]() ������̼ԭ�����ԭ�ӵľ���
������̼ԭ�����ԭ�ӵľ���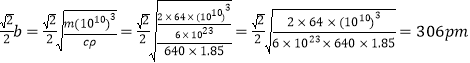
�ʴ�Ϊ��260��306��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и������ʻ�Ϊͬϵ�����
A.12C��14CB.���ʯ��ʯīC.C2H6��C3H8D.CH3CH2OH��CH3OCH3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������Ʊ���Ϊ��ҵ�Σ���Ư�ס���Ƶȷ���Ӧ�ù㷺��ijС���Ʊ��������Ƶ�ʵ��װ����ͼ��ʾ(���ּг�װ������ȥ)��
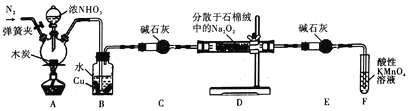
��֪��
��2NO+Na2O2=2NaNO2��2NaNO2+O2=2NaNO3
��3NaNO2+3HCl=3NaCl+HNO3+2NO��+H2O
�����������£�NO��NO2������MnO4-��Ӧ����NO3-��Mn2+
�ش��������⣺
(1)��Ӧǰ���ȴ��ɼУ�ͨ�뵪���ž�װ���еĿ�����ԭ����__________________��
(2)װ��A�з�Ӧ�Ļ�ѧ����ʽΪ_________________��װ��B�е�ʵ������Ϊ_____________��ͭƬ���ܽⲢ�����ݲ�����
(3)��ʡ��װ��C����װ��D�еĹ�������NaNO2��NaOH�⣬����__________��___________(�ѧʽ)����������к���NaNO2�ķ����ǣ�ȡ������Ʒ���Թ��У�_____________________����˵�������к���NaNO2��
(4)װ��E��������___________________________��
(5)NaNO2����ʳ��һ������ζ�����������ж�����֪���������ܷ������·�Ӧ��2NaNO2+4HI��2NO+I2+2NaI+2H2O������������Ӧ���������Լ��������г��������ʽ���ʵ�飬�Լ����������ƺ�ʳ�Ρ�����ʵ��ʱ������Ʒ�����ѡ�õ�������_________(����)��
a.�� b.�⻯�ء�������Һ c.�״� d.����
(6)��ַ�Ӧ��С�����ʵ���������NaNO2�ĺ�������ȡװ��D�й���2.3000g����ȫ�ܽ����Ƴ���Һl00mL��ȡ��25.00mL��Һ��0.100mol��L-1����KMnO4��Һ���еζ�(���ʲ���KMnO4��Ӧ)�����εζ�ƽ������KMnO4��Һ30.00mL������Ʒ��NaNO2����������Ϊ_______________��(��֪��NaNO2��Ħ������Ϊ69g��mol-1)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ĩ״��ƷA���ɵ����ʵ�����MgO��Al2O3�볣������������B��ɵĻ���A����ͼ��ʾ��ת����ϵ��
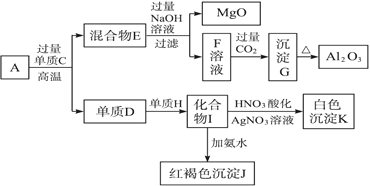
������������⣺
(1) ��������C�ͷǽ�������H�Ļ�ѧʽ�ֱ���__________��_______________
(2) д����I����J�����ӷ���ʽ��___________________
(3) д����F����G�����ӷ���ʽ��____________________________
(4) ��10.7 g��ƷA��MgO��Al2O3��B�����ʵ�����Ϊ0.05 mol����B�Ļ�ѧʽΪ___________��
(5) ��B�н���ԭ������ԭ�ӵ�������֮��Ϊ2��3��ȡ7.10 g��ƷAǡ���뺬x mol HCl��������ȫ��Ӧ����x��ȡֵ��Χ��___________(����С�������λ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��9.03��1023��CH4�У���____mol̼ԭ�ӣ�____mol��ԭ�ӣ�____mol���ӣ�____mol���ӡ���____��NH3������ͬ��Ŀ����ԭ�ӡ���״����CH4��ռ�����Ϊ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼװ�ã������Լ�bΪŨ���ᣬ������ʵ������ܴﵽʵ��Ŀ�ĵ���
ѡ�� | �Լ�a | �Լ�c | ʵ��Ŀ�� |
|
A | MnO2��NaCl | ��ɫʯ����Һ | ��֤Cl2��Ư���� | |
B | ���� | ����ʯ��ˮ | ��֤CO2������ | |
C | FeS | ��ˮ | ��֤�ǽ����ԣ�Br>S | |
D | Na2SO3 | Ʒ����Һ | ��֤SO2��Ư���� |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ѧϰ���о���ѧ��һ����Ҫ���������з���������ǣ�������
A.K2CO3��K2O��������
B.H2SO4��NaHSO4��������
C.KOH��NH3H2O�����ڼ�
D.Na2O��Na2SiO3������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͭ����Ҫ������Cu�Ļ������ڿ�ѧ�о���ҵ�����о���������;����CuSO4��Һ���������Һ�����Һ�ȡ���ش��������⣺
��1��CuSO4���ɽ���ͭ��Ũ���ᷴӦ�Ʊ����÷�Ӧ�Ļ�ѧ����ʽΪ___________��
��2��CuSO4��ĩ����������һЩ�л����е���ˮ�֣���ԭ����_______��
��3��SO42-�����幹����________������Sԭ�ӵ��ӻ����������_______��
��4��Ԫ�ؽ�Au���������ڱ��еĵ������ڣ���Cuͬ�壬Auԭ�����������Ų�ʽΪ______��һ��ͭ�Ͻ�������������ܶѻ��Ľṹ���ھ�����Cuԭ�Ӵ������ģ�Auԭ�Ӵ��ڶ���λ�ã���úϽ���Cuԭ����Auԭ������֮��Ϊ_______���þ����У�ԭ��֮�����������________��
��5������������д���ܣ���ԭ�ӿɽ��뵽��Cuԭ����Auԭ�ӹ��ɵ��������϶�С�����Cuԭ����Auԭ�ӵ�ͬ�������þ��崢���ľ����ṹΪCaF2�Ľṹ���ƣ��þ��崢���Ļ�ѧʽӦΪ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����л�Ϊͬ���칹�����
A. ���Ͱ��� B. 16O��18O C. ����Ͷ��� D. CH3OCH3��CH3CH2OH
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com