
����Ŀ��X��Y��Z��R��M��ԭ������������������Ԫ�أ���̬Xԭ�ӵ�s��������p��������3���������ڱ���Y��Z�����ҵ�һ������Y>Z��Z��Rͬ���壬M���������29���˶�״̬����ش��������⣺
��1����̬M2+�ĵ����Ų�ʽΪ__________��X��Y��Z��M�ĵ縺���ɴ�С��˳��Ϊ__________��
��2��������XCl3������ԭ���ӻ�����Ϊ__________��YCl3�����幹��Ϊ__________��XCl3��YCl3���������ڷǼ��Է��ӵ���__________��
��3��H2Z��H2Z2��H2R�ķе�ֱ�Ϊ100����158����-60.4�����Խ��ʹ�������____________��
��4��H2RO3��K1��K2�ֱ�Ϊ1.54��10-2��1.02��10-7�����ڻ�״̬��RO3���ܵ����Na2O�ܵ��磬����ݽṹ�����ʵĹ�ϵ�����������⣺
��K1>K2��____________��
��RO3���ܵ����Na2O�ܵ��磺____________��
��5��Z��M�γɵ�һ�ֻ����ᄃ����ͼ��ʾ��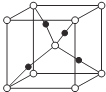 (ʵ�������M)
(ʵ�������M)
���û�����Ļ�ѧʽΪ_______________��
������������Ϊapm����ʽ����þ������ܶ���=____g��cm-3��
���𰸡�[Ar]3d9 O>N>B>Cu sp2 ������ BCl3 H2O2��H2O���Ӽ�����γ������H2O2��Է����������H2S���Ӽ䲻���γ���� SO32-��H+��Ľ������ǿ��HSO3-��H+��Ľ����������H2SO3��HSO3-����������ӣ�����̶ȴ�ӦKֵ�� SO3�ǹ��ۻ�����ǵ���ʶ�Na2O�����ӻ����� Cu2O ![]()
��������
X��Y��Z��R��M��ԭ������������������Ԫ�أ�M���������29���˶�״̬����MΪCu����̬Xԭ�ӵ�s��������p��������3����ԭ�Ӻ�������Ų�ʽΪ1s22s22p1����XΪBԪ�أ������ڱ���Y��Z�����ҵ�һ������Y>Z��Z��Rͬ���壬��Y���ڢ�����A�壬���ԭ��������֪����Y���ڢ�A�壬R��ԭ����������Cu�����������⣬��YΪNԪ�ء�ZΪOԪ�ء�RΪSԪ�ء�
��1��Cu���ڵ�������IB�壬Cu2+ԭ�Ӻ���3d�����9�����ӣ���̬Cu2+�ĵ����Ų�ʽΪ[Ar]3d9���ǽ�����Խǿ���縺��Խ�ʵ縺�ԣ�O>N>B>Cu���ʴ�Ϊ��[Ar]3d9��O>N>B>Cu����2��������BCl3��Bԭ�ӹµ��Ӷ���=![]() =0���۲���Ӷ���=3+0=3��Bԭ�Ӳ�ȡsp2�ӻ���Ϊƽ���������νṹ��������������������غϣ����ڷǼ��Է��ӣ�NCl3��Nԭ�ӹµ��Ӷ���=
=0���۲���Ӷ���=3+0=3��Bԭ�Ӳ�ȡsp2�ӻ���Ϊƽ���������νṹ��������������������غϣ����ڷǼ��Է��ӣ�NCl3��Nԭ�ӹµ��Ӷ���=![]() =1���۲���Ӷ���=3+1=4��Ϊ�����νṹ������������������IJ��غϣ����ڼ��Է��ӣ��ʴ�Ϊ��sp2�������Σ�BCl3��
=1���۲���Ӷ���=3+1=4��Ϊ�����νṹ������������������IJ��غϣ����ڼ��Է��ӣ��ʴ�Ϊ��sp2�������Σ�BCl3��
��3��H2O2��H2O���Ӽ�����γ������H2O2��Է����������H2S���Ӽ䲻���γ�������ʹ�������ķе���ߣ�ˮ��֮���������ͣ��ʴ�Ϊ��H2O2��H2O���Ӽ�����γ������H2O2��Է����������H2S���Ӽ䲻���γ������
��4����SO32-��H+��Ľ������ǿ��HSO3-��H+��Ľ����������H2SO3��HSO3-����������ӣ�����̶ȴ�ӦKֵ��
�ʴ�Ϊ��SO32-��H+��Ľ������ǿ��HSO3-��H+��Ľ����������H2SO3��HSO3-����������ӣ�����̶ȴ�ӦKֵ��
��SO3�ǹ��ۻ�������ڷǵ���ʣ���Na2O�����ӻ��������״̬�¿��Ե��磬�ʴ�Ϊ��SO3�ǹ��ۻ�������ڷǵ���ʣ���Na2O�����ӻ����
��5���پ����к�ɫ��Ϊ4����ɫ����ĿΪ1+8��![]() =2��ԭ����Ŀ֮��Ϊ2��1����Cu��O�γɵĻ�����ΪCu2 O���ʴ�Ϊ��Cu2O��
=2��ԭ����Ŀ֮��Ϊ2��1����Cu��O�γɵĻ�����ΪCu2 O���ʴ�Ϊ��Cu2O��
�ھ�������Ϊ2��![]() g�����ܶ�Ϊ2��
g�����ܶ�Ϊ2��![]() g�£�a��10-10cm��3=
g�£�a��10-10cm��3=![]() gcm-3���ʴ�Ϊ��
gcm-3���ʴ�Ϊ��![]() ��/p>
��/p>
�����硿
�ɼ۲���������жϷ������幹�ͣ��ж�ʱ��ע���������㣺��1���۲���ӶԻ���ģ��˵�����Ǽ۲���ӶԵ����幹�ͣ������ӵ����幹��ָ���dzɼ����ӶԵ����幹�ͣ��������µ��Ӷԡ���������ԭ���µ��Ӷ�ʱ�����ߵĹ���һ�£���������ԭ���йµ��Ӷ�ʱ�����ߵĹ��Ͳ�һ�¡���2���۲���ӶԻ���ģ����Ԥ����ӵļ��ι��ͣ������ܽ��ͷ��ӵijɼ�������ӻ���������ܽ��ͷ��ӵijɼ������������Ԥ����ӵļ��ι��͡��������ϣ�����һ���Ļ����ԣ��ɴﵽ���������㡢Ѹ�١�ȫ���Ч����


 �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��W��Ϊ������Ԫ�أ�������Ԫ�����ڱ��е����λ����ͼ��ʾ����Zԭ�ӵ������������ǵ�һ���������3��������˵������ȷ����( )

A. X�������̬�⻯���ˮ��Һ������
B. ����������Ӧˮ���������W��Zǿ
C. Z�ĵ�����������Ӧ��Y������������Ӧ����
D. X��ԭ�Ӱ뾶С��Y
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ɱ��400 ���ܱ������У�һ������SO2��O2�ڴ��������·�����Ӧ��2SO2(g)+O2(g) 2SO3(g)+QkJ����������������ǣ� ��
2SO3(g)+QkJ����������������ǣ� ��
A.����ѹǿ������Ӧ����һֱ���������䣬ƽ������
B.�����¶ȣ�����Ӧ���ʱ��淴Ӧ���ʼ�С�ij̶�С
C.��������������������ƽ�������ƶ���ƽ�ⳣ��Kֵ����
D.����������ʵ�������0.5molʱ�ﵽƽ�⣬��������·�Ӧ�ų�0.5QkJ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ڷ�Ӧ��4CO(g)+2NO2(g)=4CO2(g)+N2(g����H=-1200KJ/mol���¶Ȳ�ͬ(T2>T1������������ͬʱ������ͼ����ȷ����

A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪A��B��C��D����Ԫ�ص�ԭ������֮�͵���36.A�ĵ�������������壻B�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ�D�С����������֮�ƣ��䵥�ʺͻ������й㷺����;��D4+���Ӻ��ԭ�ӵĺ�������Ų���ͬ.��ҵ������DO2��̼�ᱵ������״̬����ȡ�������(�ɿ���һ�ֺ�������).��������������ġ�ѹ�����ܡ���Ӧ���ڳ������ķ���װ��.��X���߷��������������ľ����ṹΪ������(����ͼ��ʾ)������Ba2+ռ������λ�ã�O2-ռ������λ�ã�D4+ռ�ݶ���λ��.
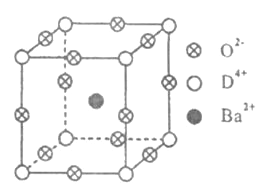
��ش��������⣺
��1��A��B��C����Ԫ�صĵ縺���ɴ�С��˳����__________________(��Ԫ�ط���).
��2��BA4���ӵĿռ乹����______________��Bԭ�ӹ�����ӻ�����Ϊ_____.
��3��C����̬�⻯��ĵ���ʽΪ____����е����ͬ��������Ԫ���⻯��ķе㣬��Ҫԭ����____________________.
��4��D�Ļ�̬ԭ�Ӻ�������Ų�ʽΪ____________________.
��5�����Ʊ�������Ļ�ѧ����ʽΪ____________________.
���ڼ����У�����D4+��������������ģ�Ba2+����������Ķ��㣬��O2-�����������__________.
���ڼ����У�D4+������λ��Ϊ__________.
����֪�����Ħ������ΪM g/mol���侧���߳�Ϊ4.03��10-10m���������ܶ�Ϊ__________________g/cm3(Ҫ���г���ʽ�������ӵ�������NA��ʾ).
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�������£����ܱ������м���һ������C(s)��H2O(g)�������淴ӦC(s) + H2O(g)![]() CO(g) + H2(g)����Ӧ�ﵽƽ���C(s)��H2O(g)ת����Ϊ2:1��ϵ��������˵����ȷ����
CO(g) + H2(g)����Ӧ�ﵽƽ���C(s)��H2O(g)ת����Ϊ2:1��ϵ��������˵����ȷ����
A.��ʼ�����C(s)��H2O(g)���ʵ���Ϊ2:1
B.������ѹǿ��H2O(g)ת���ʲ��仯
C.�ﵽƽ���������ƽ��ʽ������Ϊ16
D.��ʼ��Ӧ��������ƽ��ʽ��һֱ��С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����10 L�ܱ������У�1 mol A��3 mol B��һ�������·�Ӧ�� A(g)��xB(g)![]() 2C(g)��2 min��Ӧ�ﵽƽ��ʱ����û�����干3.4 mol������0.4 mol C�������м�������ȷ����
2C(g)��2 min��Ӧ�ﵽƽ��ʱ����û�����干3.4 mol������0.4 mol C�������м�������ȷ����
A. ƽ��ʱ�����ʵ���֮��n(A)��n(B)��n(C)��2��11��4
B. xֵ����3
C. A��ת����Ϊ20%
D. B��ƽ����Ӧ����Ϊ0.4 mol/(L��min)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ԭ�������ĵ��������ֶ�����Ԫ�أ�����ĸx��y��z������ʾ����ԭ�Ӱ뾶��Դ�С��������ۻ�����۵ı仯����ͼ��ʾ��
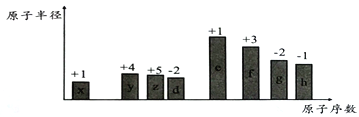
��ش��������⣺
��1��f�����ڱ��е�λ��Ϊ______________��x�γɵ������ӵĽṹʾ��ͼΪ_____________________��
��2���Ƚ�d��e�������ӵİ뾶��С��__________________���ѧʽ����ͬ�����Ƚ�g��h������������Ӧˮ���������ǿ����_______________________��
��3��x��y��z��d����Ԫ�����γɶ��ֻ����
�����γ����ӻ��������һ��x��y��z��d����ԭ�ӵĸ�����Ϊ5��2��1 ��4���仯ѧʽΪ__________________________��
�����γɹ��ۻ����д������һ�ֵĽṹ��ʽ____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ����������ͼװ����ȡ��ˮA1C13(183������������ʪ������������������)������˵����ȷ����
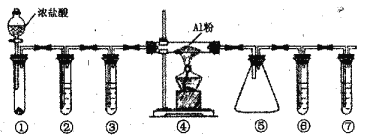
A. �����Թ���ʢװ�������̣����ڳ������Ʊ�����
B. �����������������Թ�������ʢװŨH2SO4������ʳ��ˮ��ŨH2SO4��NaOH��Һ
C. �μ�Ũ�����ͬʱ��ȼ���ľƾ���
D. �������ռ�AlCl3�������߿�����һ��װ�м�ʯ�ҵĸ���ܴ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com