
����Ŀ����֪��CH3CH2CH2CH3(g)��6.5O2(g)![]() 4CO2(g)��5H2O(l)��H=��2878kJ/mol
4CO2(g)��5H2O(l)��H=��2878kJ/mol
(CH3)2CHCH3(g)��6.5O2(g)![]() 4CO2(g)��5H2O(l)��H=��2869kJ/mol
4CO2(g)��5H2O(l)��H=��2869kJ/mol
����˵����ȷ���ǣ� ��
A.��������Ӵ�������������춡�����
B.��������ȶ��Դ����춡��
C.�춡��ת��Ϊ������Ĺ�����һ�����ȹ���
D.�춡������е�̼�����������Ķ�
���𰸡�A
��������
��֪����CH3CH2CH2CH3(g)��6.5O2(g)![]() 4CO2(g)��5H2O(l) H=��2878kJ/mol��
4CO2(g)��5H2O(l) H=��2878kJ/mol��
��(CH3)2CHCH3(g)��6.5O2(g)![]() 4CO2(g)��5H2O(l) H=��2869kJ/mol�����ݸ�˹���ɣ���-�ڿɵã�CH3CH2CH2CH3(g)
4CO2(g)��5H2O(l) H=��2869kJ/mol�����ݸ�˹���ɣ���-�ڿɵã�CH3CH2CH2CH3(g) ![]() (CH3)2CHCH3(g) H=��9kJ/mol����������ת��Ϊ�춡��Ϊ���ȷ�Ӧ���ݴ˽��
(CH3)2CHCH3(g) H=��9kJ/mol����������ת��Ϊ�춡��Ϊ���ȷ�Ӧ���ݴ˽��
��֪����CH3CH2CH2CH3(g)��6.5O2(g)![]() 4CO2(g)��5H2O(l) H=��2878kJ/mol��
4CO2(g)��5H2O(l) H=��2878kJ/mol��
��(CH3)2CHCH3(g)��6.5O2(g)![]() 4CO2(g)��5H2O(l) H=��2869kJ/mol�����ݸ�˹���ɣ���-�ڿɵã�CH3CH2CH2CH3(g)
4CO2(g)��5H2O(l) H=��2869kJ/mol�����ݸ�˹���ɣ���-�ڿɵã�CH3CH2CH2CH3(g) ![]() (CH3)2CHCH3(g) H=��9kJ/mol����������ת��Ϊ�춡��Ϊ���ȷ�Ӧ��
(CH3)2CHCH3(g) H=��9kJ/mol����������ת��Ϊ�춡��Ϊ���ȷ�Ӧ��
A. �����Ϸ���֪��������ת��Ϊ�춡��Ϊ���ȷ�Ӧ�����������غ�֪������������������춡���������A����ȷ��
B. ����������������춡�������������Խ�ͣ�����Խ�ȶ���������������ȶ���С���춡����ȶ��ԣ�B�����
C. �����Ϸ���֪���춡��ת��Ϊ������Ĺ�����һ�����ȹ��̣�C�����
D. ��������춡����ͬ���칹�壬���Ժ��е���ԭ������ͬ����̼�����ͬ��D�����
��ѡA��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���������������ʵ���Ũ�ȵ�NH4HCO3��NaCl��Һ��ϣ���������NaHCO3���壬���ˣ�������ҺpH<7�����й�����Һ�е�����Ũ�ȹ�ϵ����ȷ����
A. ![]() <1.0��10-7mol/L
<1.0��10-7mol/L
B. c(Na+)= c(HCO3��)+ c(CO32��)+ c(H2CO3)
C. c(H+)+c(NH4+)= c(OH��)+ c(HCO3��)+2 c(CO32��)
D. c(Cl��)> c(NH4+)> c(HCO3��)> c(CO32��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������װ�û������ȷ���ܴﵽʵ��Ŀ��(��Ҫ�ļг�װ�ü�ʯ������ʡ��)����(����)
A.ʵ��������ϩ
B.ʵ��������Ȳ����֤��Ȳ�ܷ���������Ӧ
C.ʵ�����з���ʯ��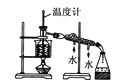
D.����Ϊ���ᣬ��Ϊ����(��״����Ҫ�ɷ�̼���)����Ϊ��������Һ����֤���ᡢ̼�ᡢ�������Ե�ǿ���������Ǵ���Ļӷ���

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����4 mol A�����2 mol B������2 L�������л�ϲ���һ�������·������·�Ӧ��2A������+B������![]() 2C������������2 s���룩����C��Ũ��Ϊ0.6 mol��L��1���������м���˵����
2C������������2 s���룩����C��Ũ��Ϊ0.6 mol��L��1���������м���˵����
��������A��ʾ�ķ�Ӧƽ������Ϊ0.3 mol��L��1��s��1
��������B��ʾ�ķ�Ӧ��ƽ������Ϊ0.6 mol��L��1��s��1
��2sʱ����A��ת����Ϊ70%
��2sʱ����B��Ũ��Ϊ0.7 mol��L��1
������ȷ���ǣ�
A.�٢�B.�٢�C.�ڢ�D.�ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����25�������½�pH=11�İ�ˮϡ��100������Һ��pHΪ������ţ�__��
A��9 B��13 C��11��13֮�� D��9��11֮��
��2��25��ʱ����0.1mol/L�İ�ˮ�м��������Ȼ�粒��壬�������ܽ�����ҺpH��С����Ҫԭ���ǣ�����ţ�_______________��
A����ˮ���Ȼ�立�����ѧ��Ӧ B���Ȼ����Һˮ�������ԣ�������c(H+)
C���Ȼ������ˮ���������������ӣ������˰�ˮ�ĵ��룬ʹc(OH�D)��С
��3�������£������0.1mol NH4Cl��0.05mol NaOHȫ������ˮ���γɻ����Һ(��������ʧ)��
��_______________��______________�������ӵ����ʵ���֮�͵���0.1mol��
��_______________��______________�������ӵ����ʵ���֮�ͱ�OH�D��0.05mol��
��4����֪ij��Һ��ֻ����OH����H+��NH4+��Cl���������ӣ�ijͬѧ�Ʋ����Һ�и�����Ũ�ȴ�С˳��������������ֹ�ϵ��
A��c(Cl��)��c(NH4+)��c(H+)��c(OH��) B��c(Cl��)��c(NH4+)��c(OH��)��c(H+)
C��c(Cl��)��c(H+)��c(NH4+)��c(OH��) D��c(NH4+)��c(Cl��)��c(OH��)��c(H+)
������Һ��ֻ�ܽ���һ�����ʣ������ʵ�������_________����������Ũ�ȴ�С˳���ϵ����ȷ���ǣ�ѡ����ţ�________________________��
����������ϵ��C����ȷ�ģ�����Һ�����ʵĻ�ѧʽ��__________________________��
��������Һ���������ȵ�ϡ����Ͱ�ˮ��϶��ɣ���ǡ�ó����ԣ�����ǰc��HCl��������>������<��������=������ͬ��____________c��NH3��H2O������Ϻ���Һ��c��NH4+����c��Cl�����Ĺ�ϵc��NH4+��___________c��Cl������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪���ȼ��a g��Ȳ����ʱ����1 mol������̼�����Һ̬ˮ�����ų�����b kJ������Ȳȼ�յ��Ȼ�ѧ����ʽ��ȷ����
A. 2C2H2(g)+5O2(g)=4CO2(g)+2H2O(l)����H=+b kJ/mol
B. C2H2(g)+![]() O2(g)=2CO2(g)+H2O(l)����H=+2b kJ/mol
O2(g)=2CO2(g)+H2O(l)����H=+2b kJ/mol
C. 2C2H2(g)+5O2(g)=4CO2(g)+2H2O(l)����H=��2b kJ/mol
D. 2C2H2(g)+5O2(g)=4CO2(g)+2H2O(l)����H=��4b kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����λ�ڢ�A�壬��±���������ʽṹ��ѧ���о��ȵ㣬Ҳ����Ҫ�Ļ���ԭ�ϡ����Ȼ���(BCl3)��������ȡ������(B2H6)��Ҳ�����л��ϳɵĴ�����
[��������]����BCl3���۵�Ϊ��107.3 �棬�е�Ϊ12.5 �棻��2B��6HCl![]() 2BCl3����3H2���������������������ƣ�Ҳ��������������Һ��Ӧ��
2BCl3����3H2���������������������ƣ�Ҳ��������������Һ��Ӧ��
[���ʵ��]��ijͬѧ�����ͼ��ʾװ���Ʊ����Ȼ���

��ش��������⣺
��1�������£����ø��������Ũ���ᷢ���ķ�Ӧ�����Aװ���еķ�Ӧ����д�����������Ũ���ᷴӦ�����ӷ���ʽ��_______________________________________________��
��2��Eװ�õ�������________________________________�������ȥBװ�ã����ܵĺ����______________________________________________________________________��
��3�����Ȼ�����ˮ���ҷ�Ӧ��������(H3BO3)�Ͱ�����д���÷�Ӧ�Ļ�ѧ����ʽ______________________________________________________��ʵ���ұ������Ȼ����ע��������___________________________________��
��4��ʵ���п�����һ��ʢװ____________________(���Լ�����)�ĸ���ܴ���F��Gװ�ã�ʹʵ�����㡣
��5��Ϊ��˳�����ʵ�飬��ȷ�IJ�����_____(�����)��
���ȵ�ȼA���ƾ��ƣ����ȼD���ƾ��� ��ͬʱ��ȼA��D���ƾ��� ���ȵ�ȼD���ƾ��ƣ����ȼA���ƾ���
��6�����㲹����������ļ���ʵ�飬����֤�ƵõIJ�Ʒ���Ƿ�����ۣ�ȡ������Ʒ���Թ��У��μ�Ũ________________(�ѧʽ)��Һ���������ݲ���������Ʒ�к�����ۣ��������ݲ���������Ʒ������ۡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵������ȷ���ǣ� ��
A.25��ʱij��Һ��ˮ�������c(H+)��1.0��1012 mol��L1����pHһ����12
B.ij�¶��£���ˮ��ͨ��CO2������CO2��ͨ�룬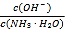 ��������
��������
C.���º����£���ӦX(g)��3Y(g) ![]() 2Z(g)����ʼ����3 mol X��3 mol Y����X�������������ʱ����Ӧ�ﵽƽ��
2Z(g)����ʼ����3 mol X��3 mol Y����X�������������ʱ����Ӧ�ﵽƽ��
D.ij�¶��£���pH��6������ˮ�м���NaHSO4���壬�����¶Ȳ��䣬�����Һ��pHΪ2�����¶��¼�������pH��10��NaOH��Һ��ʹ��Ӧ�����Һǡ�ó�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���¶��£���1L�ܱ������м���1 mol HI(g)��������Ӧ2HI(g) ![]() H2(g)+I2(g)��H2���ʵ�����ʱ��ı仯��ͼ��ʾ��
H2(g)+I2(g)��H2���ʵ�����ʱ��ı仯��ͼ��ʾ��

��1��0~2 min�ڵ�ƽ����Ӧ����v(HI)=______________________�� ���¶��£�H2(g)+I2(g) ![]() 2HI(g)��ƽ�ⳣ��K=________��
2HI(g)��ƽ�ⳣ��K=________��
��2����ͬ�¶��£�����ʼ����HI(g)�����ʵ�����ԭ����2������_________ԭ����2����
a��ƽ�ⳣ�� b��HI��ƽ��Ũ��
c���ﵽƽ���ʱ�� d��ƽ��ʱH2���������
��3��ʵ������Zn��ϡ������ȡH2����Ӧʱ��Һ��ˮ�ĵ���ƽ��________�ƶ�������������ҡ������������������������Լ��е�_______������H2�����ʽ�����
a��NaNO3 b��CuSO4 c��Na2SO4 d��NaHSO3
��4��ij������ȼ�ϵ����CsHSO4����Ϊ����ʴ���H+��������ṹ��ͼ������ܷ�Ӧ�ɱ�ʾΪ2H2+O2===2H2O�������й�˵����ȷ����___________

A������ͨ�����·��b������a��
B��b���ϵĵ缫��ӦʽΪ��O2+2H2O+4e��=4OH��
C��ÿת��0.1mol���ӣ�����1.12L��H2
D��H+��a��ͨ�����������ʴ��ݵ�b��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com