���㣺�л���ʵ��ʽ�ͷ���ʽ��ȷ��,�й��л������ʽȷ���ļ���
ר�⣺�������������ȼ�չ���
��������1�����������غ㶨�ɣ���ѧ��Ӧǰ��Ԫ����������ȷ�����л���ȼ��ȼ��ʱһ�����������룬�ݴ˷������
��2����ͨ������ʯ��ˮ����������Ϊ̼���40g���ɴ˿�֪���л���ȼ�����ɶ�����̼�����ʵ���Ϊ��
=0.4mol����������̼������Ϊ0.4mol��44g/mol=17.6g������������40g����Һ��������0.8g�����Ը��л���ȼ�����ɵ�ˮ������Ϊ��40g-0.8g-17.6g=21.6g��ˮ�����ʵ���Ϊ��
=1.2mol��ͨ����������ͭ�����ڷ�����ӦCuO+CO
Cu+CO
2�����ɵĶ�����̼�������Ʒ�Ӧ����̼���ƺ���������Ӧ����ʽΪ��2Na
2O
2+2CO
2=2Na
2CO
3+O
2���ɴ˿�֪���ؼ�ΪCO�����������ʵ���Ϊ��
=0.4mol���ʸ��л����к�̼0.4mol+0.4mol=0.8mol������1.2mol��2=2.4mol��������0.4mol��2+0.4mol+1.2mol-0.8mol��2=0.8mol���ʸ��л�����̼�������ʵ���֮��Ϊ��0.8mol��2.4mol��0.8mol=1��3��1�����ʽΪ��CH
3O������ʽΪC
2H
6O
2��
�ڽ�Ϸ���ʽ�������л������Ʒ�Ӧ�Ĺ�ϵ���жϷ����й����Ÿ������ݴ���д�ṹ��ʽ��
�۽�Ϸ���ʽ�������л������Ʒ�Ӧ�Ĺ�ϵ���жϷ����й����Ÿ������ݴ���д�ṹ��ʽ��
���
�⣺��1���������֪�л���ȼ�պ����ֻ��CO
2��H
2O����ȫȼ������CO��CO
2��H
2O�����������к���̼����Ԫ�أ����������غ㶨�ɿ�֪��������һ������̼����Ԫ�أ���Ԫ�ز���ȷ������ͨ���ⶨ�л��PCO
2��H
2O����������C��H��Ԫ�ص�����֮�͵����л��������������л���ֻ��C��H��Ԫ�أ���C��H��Ԫ�ص�����֮��С���л��������������л���һ������C��H��O����Ԫ�أ��ʴ�Ϊ��C��H��C��H��O��
��2����ͨ������ʯ��ˮ����������Ϊ̼���40g���ɴ˿�֪���л���ȼ�����ɶ�����̼�����ʵ���Ϊ��
=0.4mol����������̼������Ϊ0.4mol��44g/mol=17.6g������������40g����Һ��������0.8g�����Ը��л���ȼ�����ɵ�ˮ������Ϊ��40g-0.8g-17.6g=21.6g��ˮ�����ʵ���Ϊ��
=1.2mol��ͨ����������ͭ�����ڷ�����ӦCuO+CO
Cu+CO
2�����ɵĶ�����̼�������Ʒ�Ӧ����̼���ƺ���������Ӧ����ʽΪ��2Na
2O
2+2CO
2=2Na
2CO
3+O
2���ɴ˿�֪���ؼ�ΪCO�����������ʵ���Ϊ��
=0.4mol���ʸ��л����к�̼0.4mol+0.4mol=0.8mol������1.2mol��2=2.4mol��������0.4mol��2+0.4mol+1.2mol-0.8mol��2=0.8mol���ʸ��л�����̼�������ʵ���֮��Ϊ��0.8mol��2.4mol��0.8mol=1��3��1�����ʽΪ��CH
3O������ʽΪC
2H
6O
2��
�ʴ�Ϊ��C
2H
6O
2��
�������ϼ����֪ag���л�������ʵ���Ϊ0.4mol��18.4g�����Ƶ����ʵ���Ϊ��
=0.8mol����1mol���л����ܺ�2mol�Ʒ�Ӧ�����Ը��л��ﺬ2���ǻ����ṹ��ʽΪ��HOCH
2CH
2OH���ʴ�Ϊ��HOCH
2CH
2OH��
��9.2g�Ƶ����ʵ���Ϊ��
=0.4mol��0.4mol�л����0.4mol�Ʒ�Ӧ����֪���л����к�һ���ǻ����ṹ��ʽΪ��CH
3-O-CH
2-OH���ʴ�Ϊ��CH
3-O-CH
2-OH��
���������⿼������ȼ�շ�ȷ���л������ʽ���л���ṹ���жϡ������ŵ����ʵȣ��Ѷ��еȣ�����ԭ���غ��ж��л���ķ���ʽ������ע���л���ȼ�����ɶ�����̼��ˮ������ȷ���Ƿ���Ԫ�أ���Ҫ���������������ж�������ȷ����



 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
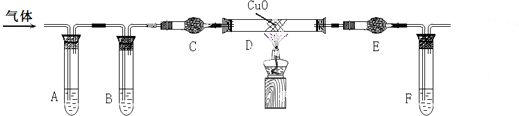
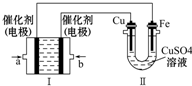 ������Ϊһ������Դ�ڻ�ѧ����Ӧ�ù㷺����ش��������⣺
������Ϊһ������Դ�ڻ�ѧ����Ӧ�ù㷺����ش��������⣺ 