
��ͼ��ʾ������ת����ϵ�У������ʾ�Ϊ����������Ԫ����ɵĵ��ʻ����֪��A��C��D��F��K��Ϊ���ʣ�C��E��F��G��K�����������壬��KΪ��ҵ������Ư�۵�ԭ��֮һ��JΪ��ɫ�����Ҽ�������B��ˮ��Һ����������E��ˮ��Һ��B��G����ʹʪ��ĺ�ɫʯ����ֽ��������Ӧ���ǹ�ҵ�ƻ��ʵ���Ҫ��Ӧ֮һ����ͼ�в��ַ�Ӧ����������δ�г���
��ش��������⣺
��1��B�ĵ���ʽΪ ��
��2��H�Ļ�ѧʽΪ ��
��3��д����Ӧ�ܵĻ�ѧ����ʽ ��
��4��д����Ӧ�ݵ����ӷ���ʽ ��
��1��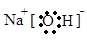
��2�� NaAlO2
��3��N2+3H2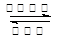 2NH3
2NH3
��4��Al3++3NH3+3H2O=Al(OH)3��+3NH4+ �� Al3++3NH3��H2O=Al(OH)3��+3NH4+
�������������KΪ��ҵ������Ư�۵�ԭ��֮һ����֪kΪ������B��G����ʹʪ��ĺ�ɫʯ����ֽ������˵��ˮ��Һ�ʼ��ԣ�GΪ������CΪ������FΪ������JΪ��ɫ�����Ҽ�������B��ˮ��Һ����������E��ˮ��Һ��˵��jΪ����������AΪ�ƣ�BΪ�������ơ�DΪ����HΪƫ�����ƣ�IΪ�Ȼ������� B�ĵ���ʽΪ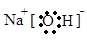 ���� H�Ļ�ѧʽΪ NaAlO2��
���� H�Ļ�ѧʽΪ NaAlO2��
�� д����Ӧ�ܵĻ�ѧ����ʽN2+3H2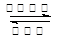 2NH3 ���� д����Ӧ�ݵ����ӷ���ʽAl3++3NH3+3H2O=Al(OH)3��+3NH4+ �� Al3++3NH3��H2O=Al(OH)3��+3NH4+��
2NH3 ���� д����Ӧ�ݵ����ӷ���ʽAl3++3NH3+3H2O=Al(OH)3��+3NH4+ �� Al3++3NH3��H2O=Al(OH)3��+3NH4+��
���㣺���⿼��������ͼ�����ʵ��ƶϡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
A��G�����ʼ�Ĺ�ϵ����ͼ��ʾ������B��DΪ���嵥�ʡ�������˵���������
A������Ӧ���ڳ����½��У���1 mol A�ڷ�Ӧ����ת��1 mol����
B����Ӧ�ڵ����ӷ���ʽΪMnO2��4H����2Cl�� Mn2����2H2O��Cl2��
Mn2����2H2O��Cl2��
C�������Ƶ�F��Һһ����Ҫ������м��ϡ���ᣬǰ�����ڷ�ֹFe2+������������Fe3+�����߿�����Fe2+��ˮ��
D����֪C��Ũ��Һ�ڴ������ڵ������¼��ȣ�����B��Ӧ����D���ɴ˿����ƶ�B�������Ա�MnO2ǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ͼת����ϵ�У��������ɫ��Ӧ�ʻ�ɫ��MΪ������Һ�����ʣ�IΪһ������������G����Ҫ�Ļ�����Ʒ�ͻ���ԭ�ϣ�����H�ܹ��ܽ���A��Һ����G�У���HΪ���õ��ͻ���ϣ�ͼ�в��ֲ���û���г�����
��1���������Һ��M��Ӧ�ķ���ʽΪ______��A��Һ�����H��Ӧ�����ӷ���ʽΪ______��
��2�������ҵĻ�ѧʽΪ_______��Һ��M�ĵ���ʽΪ_______��
��3����Ӧ�١���������������ԭ��Ӧ��Ϊ_______����д��Ӧ��ţ���
��4����I��C��ϡ��Һ����Ӧ��ֻ����G��Ũ��Һ�ڼ��������·�Ӧ����Ӧ�ߵĻ�ѧ����ʽΪ_______��
��5�����ɻ�����(FeS2)������B��Ӧ����������E����ÿ����1 mol E�ų�426.5 kJ���������÷�Ӧ���Ȼ�ѧ����ʽΪ_______ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ������A��M��һ�������µ�ת����ϵ�����ֲ��P��Ӧ����δ�г��������У�I���ɵ�������Ԫ����ɵĵ������۵���ߵĽ�����K��һ�ֺ���ɫ���塣
��ʾ��4FeS2��11O2����,2Fe2O3��8SO2
����д���пհף�
��1�������ڱ��У���ɵ���G��Ԫ��λ�ڵ�________����________�塣
��2���ڷ�Ӧ�����������뻹ԭ�������ʵ���֮��Ϊ________��
��3���ڢڡ��ۡ��ޡ����м����ڻ��Ϸ�Ӧ�����ڷ�������ԭ��Ӧ����________������ţ���
��4����Ӧ�ܵ����ӷ���ʽ��_____________________________________��
��5����������D��KNO3��KOH���ۣ����Ƶ�һ�֡���ɫ��������Ч��ˮ��K2FeO4��������أ���ͬʱ������KNO2��H2O���÷�Ӧ�Ļ�ѧ����ʽ��________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ�е��������ʾ��ɶ�����Ԫ����ɡ����мס��ҡ�������Ϊ���ʣ������¼ס���Ϊ��ɫ���壬��Ϊ����ɫ���塣���dz����������㷺���ں��졢���չ�ҵ����ҵ�ϴӺ�ˮ����ȡG����ͨ�����G��ȡ����ͬʱ�õ������ﶡ��A��E�ķ����о���10�����ӣ�A��һ����ʹʪ��ĺ�ɫʯ����ֽ���������壬E�ڳ���������ɫ��ζ����Һ����ͼ�и�����ת�����漰����������ʡ�ԡ��ش��������⣺
(1)��Ӧ�١���������������ԭ��Ӧ����_________(�����)��
(2)��������ԭ�ӽṹʾ��ͼ_________��д��A�ĵ���ʽ_________��д��A��B�ĵȵ�����(ԭ�����͵���������ȵ�����) _________��_________ (�û�ѧʽ��ʾ)��
(3)C��ˮ��Һ�����ԣ������ӷ���ʽ����_______________________________
_______________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ԫ�ؼ��仯�����������������о��й㷺����;��
��.����ѧ�ḻ���.
��1���ڳ����£����ܻ�����ϡ���ᷴӦ��������ɫ��Һ�� ��ͭ��ȣ������������ ���ǿ������������
��Cr( OH)3��Al( OH)3���ƣ�Ҳ���������������ˮ�д�����ʽ�ͼ�ʽ����ƽ�⣬����ʽ���뷽��ʽ�� ��
�ǹ�ҵ�Ͼ�����������Ⱦ����֮һ�ǣ�����K2Cr2O7���Է�ˮ���˵����ڣ�����������NaCl����Fe��ʯīΪ�缫���е�⡣����һ��ʱ�������Cr(OH)3��Fe(OH)3������ȥ(��֪KsP[ Fe(OH)3]=4.0��10-38��KsP[Cr(OH)3]=6.0��l0-31)����֪�������Һ��c( Fe3+)Ϊ2.0��10��13mol/L������Һ��c(Cr3+)Ϊ mol/L��
��.����A��H����ͼ��ʾת����ϵ(����������δ�г�)��A��E��F��G��Ϊ���壬DΪ�������ʡ�
��ش��������⣺
��1��A�ĵ���ʽΪ D�Ļ�ѧʽ ��C��Һ�������� ��
��2����Ӧ�ٵĻ�ѧ����ʽΪ ��
��Ӧ�۵����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ͼת����ϵ�У���֪B��D���ǵ���ɫ���壬��ش��������⡣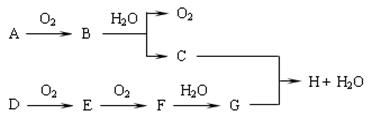
��д���������ʵĻ�ѧʽ��B ��G ��
��д�����з�Ӧ�Ļ�ѧ����ʽ��
A��B�� ��E��F�� ��B��C�� ��
�ǽ���������Eͨ�뵽���и�����Һ���������ܴ���������� ��
A��Ba2+��Ca2+��Cl�� B��OH����CO32����Na+
C��Ca2+��ClO����Cl�� D��H+��Fe3+��NO3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ͼ��ʾ������ת����ϵ�У�A�Ǻ�ˮ�к�����ḻ���Σ�B�dz�������ɫҺ�壬G��ˮ��Һ��һ�ֳ��õ�Ư����F�ǵؿ��к������Ľ���Ԫ�ء�����Ӧ�����ɵ�ˮ�Ͳ��ַ�Ӧ����δ�г���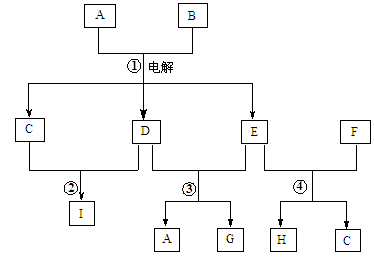
��1������A�������ӵĽṹʾ��ͼ ��
��2����Ӧ���ڵ�ȼ�����µ������� ��
��3��Hת��ΪF�������������ѡ����Լ��� ��
��4����Ӧ�۵����ӷ���ʽΪ ��
��5����Ӧ�ܵ����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ������ѧ��Ӧ����ʽΪ��A+B��X+Y+H2O��δ��ƽ����Ӧ������ȥ�������У�A��B�����ʵ���֮��Ϊ1:4����ش�
��1����YΪ����ɫ���壬�÷�Ӧ�����ӷ���ʽΪ ��B���ֳ��Ļ�ѧ������
��2����AΪ�����ķǽ������ʣ�B����ҺΪijŨ�ᣬ��Ӧ����Ϊ���ȣ��䷴Ӧ�Ļ�ѧ����ʽΪ
��3����AΪij�����õĽ������ʣ�ʵ���ҳ��ø÷�Ӧ���Ʊ�ij�����γ���������壬�÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ
��4����AΪ�����Ľ������ʣ�������A��B��Ũ��Һ�С��ۻ�������A������X��Һ�С�
��AԪ����Ԫ�����ڱ��е�λ����
�ں�amolX����Һ�ܽ���һ����A������Һ�����ֽ��������ӵ����ʵ���ǡ����ȣ���ԭ��X�����ʵ�����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com