
����ͼ��ʾ������ת����ϵ�У�A�Ǻ�ˮ�к�����ḻ���Σ�B�dz�������ɫҺ�壬G��ˮ��Һ��һ�ֳ��õ�Ư����F�ǵؿ��к������Ľ���Ԫ�ء�����Ӧ�����ɵ�ˮ�Ͳ��ַ�Ӧ����δ�г���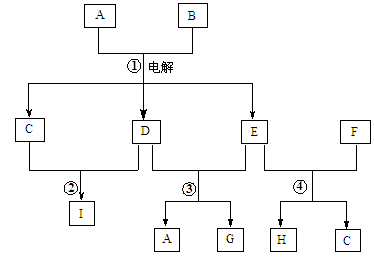
��1������A�������ӵĽṹʾ��ͼ ��
��2����Ӧ���ڵ�ȼ�����µ������� ��
��3��Hת��ΪF�������������ѡ����Լ��� ��
��4����Ӧ�۵����ӷ���ʽΪ ��
��5����Ӧ�ܵ����ӷ���ʽΪ ��
��1��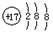 ��2�֣�
��2�֣�
��2������ȼ�ա���ɫ���桢����������2�ִ�����㼴�ɣ�
��3�� CO2��2�֣�
��4��Cl2+2OH-��Cl-+ ClO- + H2O��2�֣�
��5��2Al+2OH-+2H2O��2AlO2-+3H2����2�֣�
�������������������Ϣ����A��B��C��D��E��F��G��H�ֱ�ΪNaCl��H2O��H2��Cl2��NaOH��Al��NaClO��NaAlO2��HCl����2�������������а�����ȼ�գ�������ɫ���棬�ڻ����Ϸ�������������3��ӦΪ�����������ԣ�����ǿ��ǿ�ᷴӦ����ʵ����ƫ�����Ƶ�����������ת��Ӧ�����ᣬһ���ö�����̼��
���㣺����ͼ�����漰���ʵ��ת���й����⡣


 �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д� ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ�У�A��B��C��D��E�ǵ��ʣ�F��G��H��I��B��C��D��E�ֱ��A�γɵĻ������֪����G��H�Ļ�ѧʽΪ��X2Y3����ʽ��F�Ļ�ѧʽΪ��XY����ʽ����I��һ�ֳ��������壬��ʹ����ʯ��ˮ����ǣ���̬I�����˹����꣬ E������I��ȼ�գ������û���Ӧ����F�е�AԪ�ص���������Ϊ40%���ش����⣺

��1��I��E��Ӧ�Ļ�ѧ����ʽΪ�� ����2�֣�
��2��C���������ᷴӦ������������������Һ��Ӧ��C��A�γɵĻ�����H������������Һ��Ӧ�����ӷ���ʽΪ�� ����2�֣����H������ȡC, ��Ӧ�Ļ�ѧ����ʽΪ�� ����2�֣�
��3��G��һ�ֺ���ɫ��ĩ����һ������G�м����������ᣬ��Ӧ�Ļ�ѧ����ʽΪ�� ����2�֣���Ӧ�����Һ�м����������ۣ���Ӧ�����ӷ���ʽΪ�� ����2�֣�
��4��G�����ᷴӦ�����ӷ���ʽΪ�� ����2�֣��õ�����Һ�м���ͭ�ۣ���Ӧ�����ӷ���ʽΪ�� ����2�֣�1.6g G �������ᣬ�õ�����Һ��ͭ����ȫ��Ӧ��������ͭ�� g��3�֣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾ������ת����ϵ�У������ʾ�Ϊ����������Ԫ����ɵĵ��ʻ����֪��A��C��D��F��K��Ϊ���ʣ�C��E��F��G��K�����������壬��KΪ��ҵ������Ư�۵�ԭ��֮һ��JΪ��ɫ�����Ҽ�������B��ˮ��Һ����������E��ˮ��Һ��B��G����ʹʪ��ĺ�ɫʯ����ֽ��������Ӧ���ǹ�ҵ�ƻ��ʵ���Ҫ��Ӧ֮һ����ͼ�в��ַ�Ӧ����������δ�г���
��ش��������⣺
��1��B�ĵ���ʽΪ ��
��2��H�Ļ�ѧʽΪ ��
��3��д����Ӧ�ܵĻ�ѧ����ʽ ��
��4��д����Ӧ�ݵ����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijЩ��ѧ��Ӧ������ʽ��ʾ:A+B��C+D+H2O
��ش��������⣨��Ӧ�����ǹ���֮��ķ�Ӧ��Ҳ��������ˮ��Һ�н��еķ�Ӧ����
��1����A�Ƿǽ�����̬���ʣ���Ӧ��������Һ������������ɱ������AΪ ���ѧʽ��ͬ������Ӧ�Ļ�ѧ����ʽΪ ��
��2����AΪ�ǽ�����̬���ʣ�C��D��Ϊ�����Ҷ���ʹ����ʯ��ˮ����ǡ���AΪ ����Ӧ�Ļ�ѧ����ʽΪ ��
��3����AΪ�Ϻ�ɫ������DΪ��ɫ���塣��AΪ ����Ӧ�����ӷ���ʽΪ ��
��4����AΪ�ռ���Һ��C����Է�������Ϊ100�İ�ɫ������DΪ���Ρ���CΪ ����Ӧ�����ӷ���ʽΪ ��
��5����A��BΪ���壬C��ˮ��Һ��ʹ��̪��Һ��Ϊ��ɫ��ʵ���ҳ��ô˷�Ӧ�Ʊ�C���塣��CΪ ����Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E��F��X������ͼ��ʾת����ϵ�����У�A��һ�����Σ�B����̬�⻯�C�ǵ��ʣ�F��ǿ�ᡣX������ǿ�ᣬҲ������ǿ�
��1��A�Ļ�ѧʽ��___________________��
��2����X��ǿ�ᣬ��D��Cl2ͬʱͨ��ˮ�з�����Ӧ�����ӷ���ʽΪ_____________________________��
��3����X��ǿ�������B��Cl2��Ӧ������C�⣬��һ�������Ȼ��
�ٹ�����B��Cl2��Ӧ�Ļ�ѧ����ʽΪ______________________��
�ڹ�ҵ������B��D�Ļ�ѧ����ʽΪ_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��һ�ֺ���ɫ���������B��D�ǽ������ʣ�J��һ��������ˮ�İ�ɫ��������Ⱥ��������ֽ⡣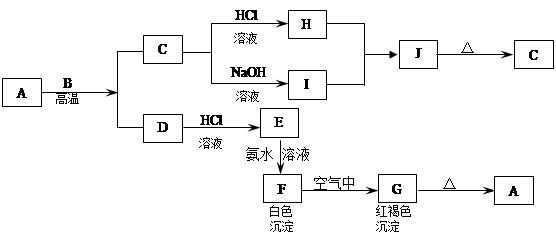
��1��A�Ļ�ѧʽ�� ��
��2��F�ڿ�����ת��ΪG�Ļ�ѧ����ʽ�� ��
��3��Cת��ΪI�����ӷ���ʽ�� ��
��4��Dת��ΪE�����ӷ���ʽ�� ��
��5��D�ڸ����º�ˮ������Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������֮���ܹ���������ͼ��ʾ�Ļ�ѧ��Ӧ���Ͻ������ֽ�����ɣ�ȡC��Һ������ɫ��Ӧ�������ʻ�ɫ���ڷ�Ӧ�в�����ˮ��δ��ͼ�б����
��1��д���������ʵĻ�ѧʽ��A ��M ��H ��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ�����������ת�Ƶ�����������ţ�
G��H ��
��3��д�����з�Ӧ�����ӷ���ʽ��
A��B+C ��
D��K ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
X��Y��Z�����ֶ�����Ԫ�أ�X��Z��������֮����Y����������ȣ�Z�ĵ��Ӳ�����X�ĵ��Ӳ�����2����A��B��C��D��E��F����ѧ��ѧ�еij������ʣ���������������Ԫ���е�һ�֡����ֻ�������ɣ�����A����ʹʪ���ɫʯ����ֽ���������壬D��E�������ᣬF��һ�ֵ��ʣ���Ӧ�ܾۢ������������½��У���ת����ϵ��ͼ��ʾ��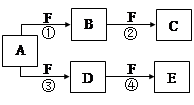
�ش��������⣺
��1��A�ĵ���ʽΪ ��
��2��A��E��Ӧ����G��C��G�ж����� ���ѧ�������ͣ���
��3����Ӧ�������ת�Ƶĵ���Ϊ3 mol����������AΪ mol��
��4����ѧ�ҷ�����ʹZX3ֱ������ȼ�ϵ�صķ�������װ���ò����缫��������Ե������Һ����һ��ͨ���������һ�缫ͨ��ZX3��ʹ֮ת��Ϊ����Ⱦ�����壬��д�������ĵ缫��Ӧʽ�� ��
��5����Z��X��Ԫ���γɵĺ�10�����ӵ������ӿɺ�XSO4���γ�һ����A������A����Һ�л�������ϡNaOH��Һ����Һǡ�ó����ԣ�����Һ����������Ũ���ɴ�С��˳��Ϊ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ҺA������ҺB�����³������ʵ�飬�������������жϣ�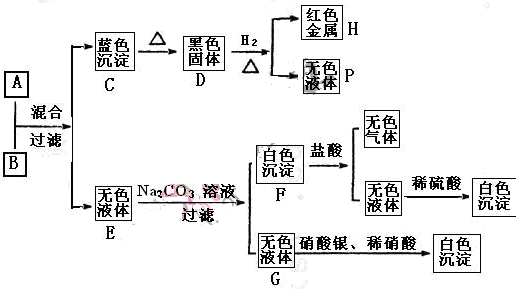
��1��A�Ļ�ѧʽ B�Ļ�ѧʽ ��
��2���������ת���Ļ�ѧ����ʽ�����á������ŷ�����������ת�Ƶķ������Ŀ��
D+H2��H+P��
��3�� д�����з�Ӧ�����ӷ���ʽ��
A��B��
F�����
��4������ҺB�������ӵļ��鷽����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com