
��ѧ������ѧ��Ӧ����ʽΪ��A+B��X+Y+H2O��δ��ƽ����Ӧ������ȥ�������У�A��B�����ʵ���֮��Ϊ1:4����ش�
��1����YΪ����ɫ���壬�÷�Ӧ�����ӷ���ʽΪ ��B���ֳ��Ļ�ѧ������
��2����AΪ�����ķǽ������ʣ�B����ҺΪijŨ�ᣬ��Ӧ����Ϊ���ȣ��䷴Ӧ�Ļ�ѧ����ʽΪ
��3����AΪij�����õĽ������ʣ�ʵ���ҳ��ø÷�Ӧ���Ʊ�ij�����γ���������壬�÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ
��4����AΪ�����Ľ������ʣ�������A��B��Ũ��Һ�С��ۻ�������A������X��Һ�С�
��AԪ����Ԫ�����ڱ��е�λ����
�ں�amolX����Һ�ܽ���һ����A������Һ�����ֽ��������ӵ����ʵ���ǡ����ȣ���ԭ��X�����ʵ�����
��1��MnO2+4H++2Cl�� Mn2++2H2O+Cl2�� ���Ժͻ�ԭ��
Mn2++2H2O+Cl2�� ���Ժͻ�ԭ��
��2��C+4HNO3(Ũ�� CO2��+4NO2��+2H2O
CO2��+4NO2��+2H2O
��3���漰��ӦΪ��Cu��4HNO3(Ũ)��Cu(NO3)2��2NO2����2H2O ���������뻹ԭ�������ʵ���֮��Ϊ 2:1
��4���ٵ������ڵڢ��� ��0��4a ����2�֡�
���������������1������ɫ����һ��Ϊ��������˷�ӦΪ��MnO2+4H++2Cl�� Mn2++2H2O+Cl2�� ���������Ӧ��������ֳ���������Ϊ�����Ժͻ�ԭ�ԡ�
Mn2++2H2O+Cl2�� ���������Ӧ��������ֳ���������Ϊ�����Ժͻ�ԭ�ԡ�
��2��C+4HNO3(Ũ�� CO2��+4NO2��+2H2O
CO2��+4NO2��+2H2O
��3��2:1
��4���������֪������Ԫ��ΪFe���������ڱ��е�λ��Ϊ���������ڵڢ���
�� Fe �� 2Fe3�� �� 3Fe2��
��ʼ��Xmol 2amol 0
��Ӧ��bmol 2bmol 3bmol
���գ���x-b��mol (2a-2b)mol 3bmol
��������ṩ������(2a-2b)= 3b������b=0��4a
��˱���ԭ��Fe3�������ʵ���Ϊ0��8a����ô����ԭ��XΪ0��4amol��
���㣺��������֮���ת����


 ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д� ��ʱ�ƿ�������ϰϵ�д�
��ʱ�ƿ�������ϰϵ�д� һ��һ��һ��ͨϵ�д�
һ��һ��һ��ͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾ������ת����ϵ�У������ʾ�Ϊ����������Ԫ����ɵĵ��ʻ����֪��A��C��D��F��K��Ϊ���ʣ�C��E��F��G��K�����������壬��KΪ��ҵ������Ư�۵�ԭ��֮һ��JΪ��ɫ�����Ҽ�������B��ˮ��Һ����������E��ˮ��Һ��B��G����ʹʪ��ĺ�ɫʯ����ֽ��������Ӧ���ǹ�ҵ�ƻ��ʵ���Ҫ��Ӧ֮һ����ͼ�в��ַ�Ӧ����������δ�г���
��ش��������⣺
��1��B�ĵ���ʽΪ ��
��2��H�Ļ�ѧʽΪ ��
��3��д����Ӧ�ܵĻ�ѧ����ʽ ��
��4��д����Ӧ�ݵ����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������֮���ܹ���������ͼ��ʾ�Ļ�ѧ��Ӧ���Ͻ������ֽ�����ɣ�ȡC��Һ������ɫ��Ӧ�������ʻ�ɫ���ڷ�Ӧ�в�����ˮ��δ��ͼ�б����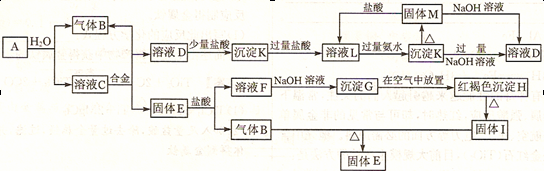
��1��д���������ʵĻ�ѧʽ��A ��M ��H ��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ�����������ת�Ƶ�����������ţ�
G��H ��
��3��д�����з�Ӧ�����ӷ���ʽ��
A��B+C ��
D��K ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
X��Y��Z�����ֶ�����Ԫ�أ�X��Z��������֮����Y����������ȣ�Z�ĵ��Ӳ�����X�ĵ��Ӳ�����2����A��B��C��D��E��F����ѧ��ѧ�еij������ʣ���������������Ԫ���е�һ�֡����ֻ�������ɣ�����A����ʹʪ���ɫʯ����ֽ���������壬D��E�������ᣬF��һ�ֵ��ʣ���Ӧ�ܾۢ������������½��У���ת����ϵ��ͼ��ʾ��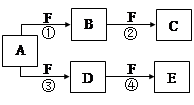
�ش��������⣺
��1��A�ĵ���ʽΪ ��
��2��A��E��Ӧ����G��C��G�ж����� ���ѧ�������ͣ���
��3����Ӧ�������ת�Ƶĵ���Ϊ3 mol����������AΪ mol��
��4����ѧ�ҷ�����ʹZX3ֱ������ȼ�ϵ�صķ�������װ���ò����缫��������Ե������Һ����һ��ͨ���������һ�缫ͨ��ZX3��ʹ֮ת��Ϊ����Ⱦ�����壬��д�������ĵ缫��Ӧʽ�� ��
��5����Z��X��Ԫ���γɵĺ�10�����ӵ������ӿɺ�XSO4���γ�һ����A������A����Һ�л�������ϡNaOH��Һ����Һǡ�ó����ԣ�����Һ����������Ũ���ɴ�С��˳��Ϊ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ������Ӧ�Ļ�ѧ����ʽ�ǣ�A+B��X+Y+H2O��δ��ƽ����Ӧ������ȥ��������A��B�����ʵ���֮��Ϊ1��4����ش�
��1����Y�ǻ���ɫ���壬�÷�Ӧ�����ӷ���ʽ�� ��
��2����AΪ�����ķǽ������ʣ�B����ҺΪijŨ�ᣬ����������ĽṹʽΪ ______
��3����AΪ�������ʣ�������A��B��Ũ��Һ�С��ۻ�������A������X��Һ�С��� a mol X����Һ�ܽ���һ����A������Һ�����ֽ��������ӵ����ʵ���ǡ����ȣ���ԭ��X�� mol��
��4����A��B��X��Y��Ϊ�������A��Һ�м��������ữ��AgNO3��Һ��������ɫ������B����ɫΪ��ɫ����A��B�����ʵ���֮��1��4��Ӧ����Һ�����ʵĻ�ѧʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾ����֪��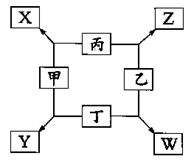
�ټס��ҡ���������Ϊǰ������Ԫ�صĵ��ʣ��ס��ҡ� ��������Ϊ���塣
����һ�������¼�����ͼ��붡����������֮��l��3��Ӧ���ֱ�����X��Y���ڲ�����Ԫ�ؼ׳ʸ��ۡ�
����һ������������������붡�������ʵ���֮��1��2��Ӧ���ֱ�����Z��W���ڲ�����Ԫ���ҳʸ��ۡ�
����գ�
��1��W�ĵ���ʽΪ��������������
��2��X���Ҵ������Ļ�ѧ����ʽ��______________________________________________��
��3��Y��Z��Ӧ�Ļ�ѧ����ʽ��_____________________________________________��
��4��2.4g�����������ҷ�Ӧ����W�ų�QkJ���ȣ���÷�Ӧ���Ȼ�ѧ����ʽΪ ��
��5��ʵ������ȡ�������ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������ת���У�A��һ�����Σ�D����Է���������C����Է���������16��E�����X������ǿ�ỹ��ǿ��ʱ���������µ�ת����ϵ��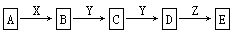
��X��ǿ��ʱA��B��C��D��E����ͬһ��Ԫ�أ���X��ǿ��ʱ��A��B��C��D��E��������ͬһ��Ԫ�ء�����֪H2CO3��H2S��H2SiO3��Ϊ��Ԫ���ᣩ
��ش�
��1��A��________��Y��________��Z��________�� ���ѧʽ����ͬ��
��2����X��ǿ��ʱ��E��________��д��B����C�Ļ�ѧ����ʽ��___________________
��3����X��ǿ��ʱ��E��________��д��B����C�Ļ�ѧ����ʽ��__________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ҺA������ҺB�����³������ʵ�飬�������������жϣ�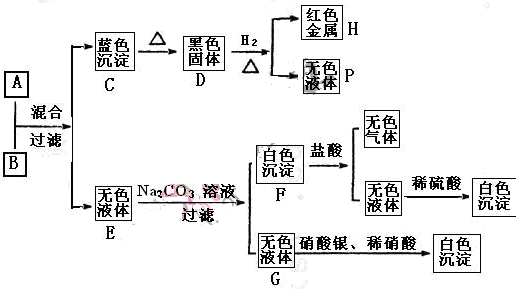
��1��A�Ļ�ѧʽ B�Ļ�ѧʽ ��
��2���������ת���Ļ�ѧ����ʽ�����á������ŷ�����������ת�Ƶķ������Ŀ��
D+H2��H+P��
��3�� д�����з�Ӧ�����ӷ���ʽ��
A��B��
F�����
��4������ҺB�������ӵļ��鷽����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
������ͼ��ʾװ����ȡ����Ҫʱ�ɼ��ȣ����������ռ��������� 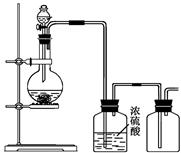
| A��ͭ��ϡ���ᷴӦ��һ������ |
| B���Ȼ�����������ƹ��巴Ӧ�ư��� |
| C��п��ϡ���ᷴӦ������ |
| D���������ƹ��������ᷴӦ�ƶ������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com