
����Ŀ�����ʵķ����ж��ַ��������ж��������������ͼ��

��1����ͼ��ʾ�����ʷ������������________��
��2����Ԫ�� Na��Ba��H��O��S��N ���������ֻ�����Ԫ����ɺ��ʵ����ʣ�����ѧʽ�ֱ������±��Тܡ��ĺ���________��__________

��3���ߺ͢��ˮ��Һ�ɵ��磬����________����ǡ����ǡ�������ʣ���ͬ������������������ԭ�ӵĸ�����Ϊ________����״���µ�����������������Ϊ________��
��4���������������ܵ���Һ��Ӧ�����ӷ���ʽΪ________��
��5��д������ᷴӦ�Ļ�ѧ����ʽ________��
��6��д����ҵ������������Т�NH3 ��������Ӧ�Ļ�ѧ����ʽΪ________���� 16g ����ȫ������ԭ����ת�Ƶ���________mol��
��7�������ɢ���ڻ�ϵ�ϡ��Һ 100mL�����Тٵ����ʵ���Ũ��Ϊ 2.0mol��L��1���ڵ����ʵ���Ũ��Ϊ 1.0mol��L��1�������Һ���ܽ�ͭ���������Ϊ________g��ͬʱ����Ӧ���ɵ������ڱ�״���µ����Ϊ________L��
���𰸡���״���෨ NaOH��Ba(OH)2 BaSO4 ���� 16:11 11:16 SO2+2OH-=SO32-+H2O��Ba2++ SO2+2OH-= BaSO3��+H2O 2CO2+2Na2O2= 2Na 2CO3+O2�� 4NH3+5O2![]() 4NO+6H2O 2 9.6 2.24
4NO+6H2O 2 9.6 2.24
��������
��1����1����״���෨��һ�ֺ�����ķ��෨������һ�ô�����
��2����������������ȫ��Ϊ���������ӣ��ε������������Ϊ�������ӣ�������Ϊ������ӣ�
��3�����ݵ���ʵĶ����жϣ�����![]() ����ͬ�����Ķ�����̼����������������ԭ�ӵĸ����ȣ���״���µ����������̼��������������ʵ�����ȣ�
����ͬ�����Ķ�����̼����������������ԭ�ӵĸ����ȣ���״���µ����������̼��������������ʵ�����ȣ�
��4������SO2����������������Һ��Ӧ�����������ƺ�ˮ��
��5��CO2��Na2O2��Ӧ����̼���ƺ�������
��6��NH3��������Ӧ����NO��H2O����Ӧ����Ԫ�ػ��ϼ���0��Ϊ-2��
��7������ͭ�����ᷴӦ�����ӷ���ʽ�����ܽ�ͭ�������������Ӧ���ɵ�����������
��1����״���෨��һ�ֺ�����ķ��෨������һ�ô�������Ҷ��֦���ˡ�����ͼʾ����������״���෨��
��2����������������ȫ��Ϊ���������ӣ���NaOH��Ba(OH)2 ���ε������������Ϊ�������ӣ�������Ϊ������ӣ���BaSO4��
��3��������������̼��ˮ��Һ���磬�����������ᡢ̼���ܵ���������ƶ������ӣ���������������̼�������ܵ��룬���Զ�����������̼���ǵ���ʣ���ͬ�����Ķ�����̼����������������ԭ�ӵĸ�����Ϊ![]() ��2��
��2��![]() ��2=16:11����״���µ����������̼��������������ʵ�����ȣ������ȵ���Ħ�������ı�=44:64=11:16��
��2=16:11����״���µ����������̼��������������ʵ�����ȣ������ȵ���Ħ�������ı�=44:64=11:16��
��4������SO2����������������Һ��Ӧ�����������ƺ�ˮ����Ӧ���ӷ���ʽΪSO2+2OH-=SO32-+H2O��
��5��CO2��Na2O2��Ӧ����̼���ƺ���������Ӧ��ѧ����ʽΪ2CO2+2Na2O2= 2Na 2CO3+O2����
��6��NH3��������Ӧ����NO��H2O����Ӧ�Ļ�ѧ����ʽΪ4NH3+5O2![]() 4NO+6H2O����Ӧ����Ԫ�ػ��ϼ���0��Ϊ-2�� 16g ���������ʵ�����0.5mol��ȫ������ԭ��ת�Ƶ���2mol��
4NO+6H2O����Ӧ����Ԫ�ػ��ϼ���0��Ϊ-2�� 16g ���������ʵ�����0.5mol��ȫ������ԭ��ת�Ƶ���2mol��
��7����Һ�������ӵ����ʵ���Ϊ0.1L��(4.0mol��L��1+1.0mol��L��1)=0.5mol��NO3-�����ʵ���Ϊ0.1L��1.0mol��L��1=0.1mol������ͭ�����ᷴӦ�����ӷ���ʽ3Cu+8H++2NO3-=3Cu2++2NO+4H2O����������Ӳ��㣬������������Ӽ����ܽ�ͭ�����ʵ���Ϊ0.15mol������Ϊ0.15mol��64g/mol= 9.6g��ͬʱ����NO����0.1mol���ڱ�״���µ����Ϊ0.1mol ��22.4L/mol=2.24L��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ��ʾװ�ý�������ʵ�飺��������Һ�������У�Ԥ���������ʵ���������

ѡ�� | �������� | �������� | Ԥ�����е����� |
A�� | ϡ���� | ̼�������������ƵĻ����Һ | ������������ |
B�� | Ũ���� | ��ɰֽ��ĥ�������� | ��������ɫ���� |
C�� | �Ȼ�����Һ | Ũ����������Һ | ����������ɫ���� |
D�� | ������Һ | �������������Һ | ��Һ����ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���۲�����ģ�Ͳ�����й���Ϣ�����жϣ�����˵����������� ��
HCN | S8 | SF6 | B12�ṹ��Ԫ | |
�ṹģ ��ʾ�� ͼ |
|
|
|
|
��ע | / | ������CS2 | / | B���۵�Ϊ1873K |
A.HCN�ĽṹʽΪH��C��N
B����̬S8����ԭ�Ӿ���
C��SF6���ɼ��Լ����ɵķǼ��Է���
D������������ԭ�Ӿ��壬��ṹ��Ԫ�к���30��B��B��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������źϽ������������Ĵ����ܶ�����Ϊ�������������������źϽ��к�������������ϡ��Ԫ���ϡ�
��1����̬��ԭ�ӵļ۲�����Ų�ͼΪ___________________����Ԫ���������ڵ�һ��������������Ԫ����________(��Ԫ�ط���)����֪��̬��(Nd)ԭ�ӵļ۲�����Ų�ʽΪ4f46s2�����̬��ԭ�Ӻ����__________�����͵��ܼ��ֲ���
��2�����������γ�����������һϵ�з��ӣ���B2H6��B3H9��B4H10�ȡ�
��B2H6��B3H9��B4H10�������ʵķе��ɵ͵��ߵ�˳��Ϊ__________________________��
��������Ľṹʽ�ɱ�ʾΪ��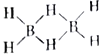 ����֪��ӦB2H6+3O2
����֪��ӦB2H6+3O2![]() B2O3+2H2O�б�������Ԫ������Ԫϵ����H��B��O����Ԫ�ص縺���ɴ�С��˳��Ϊ___________________��B2H6��������4���Ҽ���2����ͬ�Ĵ�м�(��ԭ�Ӽ乲�ö�������γɵ�һ�ֹ��ۼ�)����һ����м���ԭ�ӡ�������Ŀ�ֱ�Ϊ__________________________��
B2O3+2H2O�б�������Ԫ������Ԫϵ����H��B��O����Ԫ�ص縺���ɴ�С��˳��Ϊ___________________��B2H6��������4���Ҽ���2����ͬ�Ĵ�м�(��ԭ�Ӽ乲�ö�������γɵ�һ�ֹ��ۼ�)����һ����м���ԭ�ӡ�������Ŀ�ֱ�Ϊ__________________________��
��3����������к���������-OH����������ˮ����������ˮ����Ҫԭ����_________________��
��4��ͼ1�������������һ�����ģ��ͼ�� ����ԭ�ӡ���ԭ�ӵ��ӻ�������ͷֱ�Ϊ_____________��
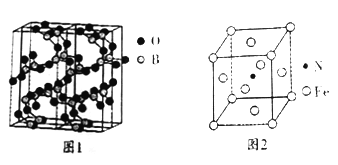
��5����Ԫ���뵪Ԫ��Ҳ���γ��ִ��Բ��ϣ��侧����ͼ2��ʾ���ô��Բ��ϵĻ�ѧʽΪ__________�����þ�����ܶ�Ϊp����ԭ�ӡ���ԭ�ӵİ뾶�ֱ�Ϊr(Fe)pm��r(N)pm����þ����Ŀռ�������Ϊ_______���谢���ӵ�������ֵΪNA����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�Ȼ�þ��Һ���ܶ�Ϊ 1.16g��cm��3������þ���ӵ���������Ϊ 4.1����500mL ����Һ�� Cl�������ʵ���Ũ��Լ���ڣ� ��
A. 4.0mol��L��1B. 2.4mol��L��1C. 2.1mol��L��1D. 1.26mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�о�С����̽��SO2�Ļ�ѧ���ʣ����������ʵ�鷽����
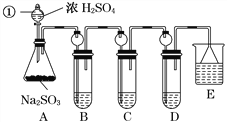
(1)ָ�������������ƣ�______________��
(2)���Aװ�õ������Եķ�����______________________________________________��
(3)װ��B����SO2�������ԣ���B����ʢ�Լ�����Ϊ________��
(4)װ��C��ʢװ��ˮ���Լ���SO2��________�ԣ���C�з�Ӧ�����ӷ���ʽΪ
________________________________________________________________________��
(5)װ��D��ʢװ����Ư��Ũ��Һ��ͨ��SO2һ��ʱ���D�г����˴�����ɫ������ͬѧ�Ƕ�ɫ�����ɷ�������ּ��裺
������һ���ð�ɫ����ΪCaSO3��
��������ð�ɫ����Ϊ__________________________________________________��
���������ð�ɫ����Ϊ�����������ʵĻ���
�����ڼ���һ��ͬѧ�Ƕ�ɫ�����ɷֽ�����̽����������·�����
��ѡ���������Լ�������װ�á��Թܡ��ιܡ������ܵĵ�����������ˮ��0.5 mol��L��1HCl��0.5 mol��L��1H2SO4��0.5 mol��L��1BaCl2��1 mol��L��1NaOH��Ʒ����Һ��
��1������D�г������ˡ�ϴ�Ӹɾ������á�
��ش�ϴ�ӳ����ķ�����____________________________________________________��
��2��������һֻ�ɾ��Թ�ȡ����������Ʒ������________(�Լ�)�����ϴ����ܵĵ������������ܵ���һ�˲���ʢ��________(�Լ�)���Թ��С�������__________________���������һ������
�����������������д�����ɸð�ɫ�����Ļ�ѧ����ʽ��_________________________________��
(6)װ��E��ʢ�ŵ��Լ���________��������__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ļ������������ᣬ�μ�KSCN��Һ��������ɫ�仯���ټ���������ˮ����Һ�����ʺ�ɫ����( )
A. FeO B. FeCl3 C. Fe2(SO4)3 D. Fe2O3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ�����ӵ�������ֵ������˵������ȷ���ǣ� ��
A. 2.8 g��ϩ�ͱ�ϩ�Ļ������������̼ԭ����Ϊ0.2 NA
B. ��״���£�2.24 L CCl4�к��е���ԭ����Ϊ0.4 NA
C. 1 mol��ϩȩ�����к��е�˫����ΪNA
D. 1 mol CH4���еĵ�����ΪNA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������װ���ܴﵽʵ��Ŀ����( )
A. ������Һʱת����Һ
B. ʯ������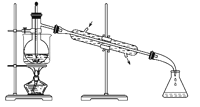
C. �۲�ص���ɫ��Ӧ
D. �Ƚ�NaHCO3��Na2CO3���ȶ���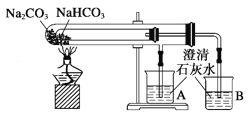
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com