
����Ŀ�������۵���ߵĽ���������Ҫ��ս�����ʡ���Ȼ��������Ҫ�����ں��ٿ��У�����Ҫ�ɷ��������̵������Σ�FeWO4��MnWO4������������Si��As�Ļ�����ɺ��ٿ�ұ���ٵĹ����������£�

��֪��
������I����Ҫ�ɷ���Fe2O3��MnO2��
�����������У��ٵĻ��ϼ�ֻ�������һ�������ı䡣
������������������ˮ��
��1����д��FeWO4�����������·�����ֽⷴӦ����Fe2O3�Ļ�ѧ����ʽ��______________________________________��
��2�����������������������Һ�м������к���pH=10����Һ�е�����������ΪSiO32�D��HAsO32�D��HAsO42�D�ȣ����������������У�����H2O2ʱ������Ӧ�����ӷ���ʽΪ_________������������Ҫ�ɷ���______________��
��3����֪�������ƺ�����ƣ�CaWO4�������ܵ���ʣ����ߵ��ܽ�Ⱦ����¶����߶���С����ͼΪ��ͬ�¶���Ca��OH��2��CaWO4�ij����ܽ�ƽ��������

��T1_____T2������>������<����T1ʱKsp��CaWO4��=______________��
������������Һ����ʯ����õ���������ƣ�������Ӧ�����ӷ���ʽΪ__________________________��
���𰸡�4FeWO4+O2+8NaOH![]() 2Fe2O3+4Na2WO4+4H2O H2O2+HAsO32�D�THAsO42�D+H2O MgSiO3 �� MgHAsO4 < 1��10-10 WO42�D+Ca(OH)2=CaWO4+2OH�D
2Fe2O3+4Na2WO4+4H2O H2O2+HAsO32�D�THAsO42�D+H2O MgSiO3 �� MgHAsO4 < 1��10-10 WO42�D+Ca(OH)2=CaWO4+2OH�D
��������
�����̿�֪�������������������ơ�������Ӧ�����������������ƣ������̺��������Ʒ�Ӧ���������ƺ��������̣�ˮ��ʱ�����������������̲�����ˮ������������ˮ���ʹ��˺�õ�����Һ�������ƣ�����I����Ҫ�ɷ���Fe2O3��MnO2�������ƺ�Ũ���ᷴӦ��������������ƣ�����������⣬����+5�۵���Ϊ+6�ۣ������Ȼ�þ������������ˮ��MgSiO3��MgHAsO4�����ˣ���ҺΪ�����ƣ��ữ�����ȷֽ�����������ٺ�ˮ���û�ԭ����ԭ�������������٣��ݴ˷������
��1����������ͼ��֪�����������������ơ�������Ӧ�����������������ƣ���Ӧ�ķ���ʽΪ4FeWO4+O2+8NaOH![]() 2Fe2O3+4Na2WO4+4H2O��
2Fe2O3+4Na2WO4+4H2O��
��2���������Ϸ���������H2O2��Ŀ���ǽ�HAsO32-������HAsO42-�����ӷ���ʽΪH2O2+HAsO32-�THAsO42-+H2O����ҺI�д���SiO32-��HAsO32-��HAsO42-�����ӣ���������pHֵ�����Ȼ�þ��Mg2+����SiO32-��HAsO32-��HAsO42-�����ӣ����������Ҫ�ɷ���MgSiO3��MgHAsO4��
��3���ٸ���ͼ���֪���������ƺ�������ڸ�����Ũ����ͬʱ��T1�¶���������Ũ�ȴ���T2��˵��T1ʱ���ܶȻ�����T2���ܶȻ�Խ�����ܽ��Խ������T1ʱ�ܽ�Ƚϴ����ڡ���֪�������ƺ�����ƣ�CaWO4�������ܵ���ʣ����ߵ��ܽ�Ⱦ����¶����߶���С������T1��T2��T1ʱKsp��CaWO4��=c��Ca2+��c��WO42-��=1��10-5��1��10-5=1��10-10��
�ڽ���������Һ����ʯ���飬�������ֽⷴӦ���������ƺ���������ӷ�Ӧ��������Ƴ�������Ӧ�����ӷ���ʽΪWO42�D+Ca(OH)2=CaWO4+2OH�D��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Na��Mg��Al�й㷺��Ӧ�á�
��1��Al��Ԫ�����ڱ��е�λ����__________________��
��2������þ�����������������ˣ�Ԫ�ط�����U����UF4+2Mg![]() U+2MgF2���÷�Ӧ�У���Ϊ��ԭ����������_________���ѧʽ����ͬ��������ԭ��������_________��
U+2MgF2���÷�Ӧ�У���Ϊ��ԭ����������_________���ѧʽ����ͬ��������ԭ��������_________��
��3��Ϊ�Ƚ�Na��Mg��Al�Ľ����ԣ�����������ʵ�飨��������ı��������ͬ����
ʵ��1 | ʵ��2 |
|
|
����ˮ��Ӧ���ң�þ��ˮ��Ӧ���� | þ�����ᷴӦ���ң��������ᷴӦ���� |
��ʵ��1��ʵ��2�ó��Ľ����ǣ�������_________>_________>_________����Ԫ�ط��ţ�����ԭ�ӽṹ���۽��ͣ�ͬ����Ԫ�ش����ң�_________��
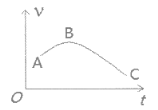
��4����þ����ȥ������Ĥ��Ͷ�뵽ʢ������ij��������У�����H2������v��ʱ��t�Ĺ�ϵ��ͼ��ʾ��AB�������������Ҫԭ����__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ�������ȷ�Ӧ����( )
A. C6H12O6(������aq)��6O2![]() 6CO2��6H2O
6CO2��6H2O
B. ����������Һ��������кͷ�Ӧ
C. ��Ӧ��������������������������
D. �ƻ���Ӧ��ȫ����ѧ���������������ƻ�������ȫ����ѧ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪����A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ����2CH3CHO��O2![]() 2CH3COOH������AΪ��Ҫԭ�Ϻϳɻ�����E����ϳ�·����ͼ1��ʾ���ش��������⣺
2CH3COOH������AΪ��Ҫԭ�Ϻϳɻ�����E����ϳ�·����ͼ1��ʾ���ش��������⣺
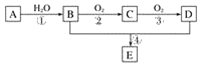
ͼ1
(1)д���������ʵĹ��������ƣ�
B��____________________��D��____________________��
(2)��Ӧ�ܵĻ�ѧ����ʽΪ________________________________________________����Ӧ���ͣ�________��
(3)ijѧϰС���������B��������ʵ��װ�����£�����ͼ2װ�ûش����⡣
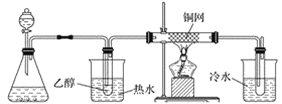
�� �� �� ��
ͼ2
��װ�ü���ƿ��ʢ�ŵĹ���ҩƷ����Ϊ________(����ĸ)��
A Na2O2 B KCl C Na2CO3 D MnO2
��ʵ������У���װ��Ӳ�ʲ������з�����Ӧ�Ļ�ѧ����ʽΪ_________________________��
������B�Ĵ����������������Ǿ�����ͬ��������Ӧ�������õ���������μӵ�����������ͭ����Һ�м��ȣ�����Ϊ______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ��ȤС��ͬѧչ����Ư����������(NaClO2)���о���
ʵ��������ȡNaClO2���塣
��֪��NaClO2������Һ���¶ȵ���38 ��ʱ�����ľ�����NaClO2��3H2O������38 ��ʱ�����ľ�����NaClO2������60 ��ʱNaClO2�ֽ��NaClO3��NaCl��������ͼ��ʾװ�ý���ʵ�顣
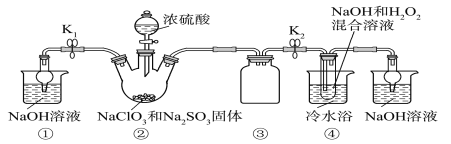
��1��װ������������_______________
��2��װ�����в���ClO2����Ļ�ѧ����ʽΪ____________��
��3����װ������Ӧ�����Һ��þ���NaClO2�IJ�������Ϊ��
����ѹ��55 �������ᾧ��
�����ȹ��ˣ�
��____________________________________��
������60 ������õ���Ʒ��
ʵ�������ⶨij����������Ʒ�Ĵ��ȡ��������ʵ�鷽����������ʵ�顣
��ȷ��ȡ��������������Ʒm g���ձ��У�������������ˮ�����ĵ⻯�ؾ��壬�ٵ���������ϡ���ᣬ��ַ�Ӧ(��֪��ClO2����4I����4H��===2H2O��2I2��Cl��)�������û��Һ���100 mL������Һ��
����ȡ25.00 mL������Һ����ƿ�У���c mol��L��1 Na2S2O3��Һ�ζ������ζ��յ㡣�ظ�2�Σ�������ı���Һ�������ƽ��ֵΪV mL(��֪��I2 ��2S2O32��===2I����S4O62��)��
��4����Ʒ��NaClO2����������Ϊ__________(�ú�m��c��V�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijʳƷ���������Ҫ�ɷ��������ƣ������ƣ�CaO��Ӧ���ڣ�������
A. �� B. �� C. �� D. ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ��ʵ����ˮ���Ρ���ij�������ۺϴ�����NH4+��ˮ��ҵ����(��Ҫ��N2��CO2��SO2��NO��CO�������������ɷ�)��������������̣�

�ش��������⣺��
(1)����1���е�������_________��CaCO3��Ca(OH)2��������������������Ҫ��_______________��
(2)��X�ǿ�������������Ӧ��NO��O2�����ʵ���֮�����Ϊ___________�����������������µĽ������____________________________��
(3)�����д�����NH4+��ˮʱ������Ӧ�����ӷ���ʽΪ____________________________������1Ҳ��ͨ������ֱ��ת��������Ⱦ���壬��Ӧ�Ļ�ѧ����ʽΪ_____________________________________��
(4)����ҵ��������Ҫ��N2��SO2�������������ɷ֣���������й�������NaHSO3��������Һ[NaHSO3��������Һ����������������(Na2S2O5)��ԭ��]��
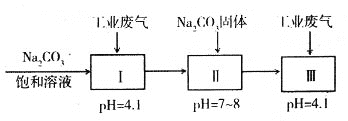
pH=4.1ʱ��I�з�Ӧ�Ļ�ѧ����ʽΪ_________________________�������м���Na2CO3
���塢���ٴ�ͨ�������Ŀ����_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ܱ������н��еķ�ӦΪ X(g)��3Y(g) ![]() 2Z(g)��X��Y��Z ����ʼŨ������Ϊ0.1 mol / L��0.3 mol / L��0.2 mol / L������Ӧ��ƽ��ʱ�������ʵ�Ũ�ȿ����� ( )
2Z(g)��X��Y��Z ����ʼŨ������Ϊ0.1 mol / L��0.3 mol / L��0.2 mol / L������Ӧ��ƽ��ʱ�������ʵ�Ũ�ȿ����� ( )
A. X��0.2 mol / L��Y��0.6 mol / L B. Y��0.5 mol / L��Y��0.1 mol / L
C. Y��0.6 mol / L D. Z��0.4 mol / L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25 ��ʱ�������ΪVa��pH��a��ijһԪ��HA��Һ�����ΪVb��pH��b��ijһԪ��BOH��Һ��ϣ���ش�
��1����a + b��14��2Va��Vb����Ӧ��������ҺpH��7�������ɵ�����Һ�У�һ������ˮ������ӷ� ��ʽΪ_______
��2����a + b��12���������ᣬ����KOH����Ӧ��������ҺpH��7����Va��Vb�Ĺ�ϵ��_______
��3�����������ᣬ���ǰ�ˮ����Ӧ��������Һ������Ũ�ȴ�С��ϵ��������_________������ţ�
A��c(Cl��)��c(NH)��c(H+)��c(OH��) B��c(H+)��c(OH��)��c(Cl��)��c(NH)
C��c(NH)��c(Cl��)��c(OH��)��c(H+) D��c(Cl��)��c(H+)��c(NH)��c(OH��)
E��c(Cl��)��c(NH)��c(H+)��c(OH��)
��4�������Ǵ��ᣬ����NaOH���ҷ�Ӧ������Һ��c(CH3COO��)��c(H+)��������Һ���ܳ�_____������ţ�
A������ B������ C������ D�������
��5��25 ��ʱ�������Va��200 mL��pH��2��H2SO4��Һ�����Vb��10 mL��pH��11�İ�ˮ��Һ��ϣ�ǡ����ȫ��Ӧ����������£���ˮ�ĵ���ƽ�ⳣ����____________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com