��������1������ȼ����д���Ȼ�ѧ����ʽ�����ø�˹���ɼ��㣻
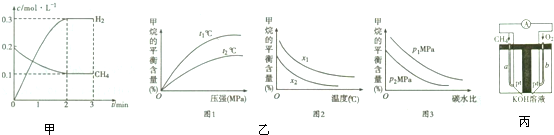
��2������ͼ���֪3minʱ��ƽ��ʱ����ֵ�ƽ��Ũ�ȣ��ٸ���4minʱ�����Ũ�ȱ仯���жϸı��������
��3���ٸ��ݷ�Ӧ����ЧӦ�ж��¶ȶ�ƽ���ƶ���Ӱ�죬���ͼ��������
��̼ˮ��
ֵԽ��ƽ��ʱ�����ת����Խ�ͣ�����Խ�ߣ�
�۸���ѹǿ��ƽ���ƶ�Ӱ�죬���ͼ��������
��4��������������ԭ��Ӧ�������������ŵ��������������ӣ�
�ڼ������������ʵ����������������ɶ�����̼�����ʵ���������n��NaOH����n��CO
2��������ϵ�жϷ�Ӧ�������������Һ�е�������ʵ������������ˮ���������жϣ�
���
�⣺��1����֪����H
2��g��+
O
2��g��=H
2O��l����H=-285.8kJ?mol
-1��CO��g��+
O
2��g��=CO
2��g�� ����H=-283.0kJ?mol
-1��CH
4��g��+2O
2��g��=CO
2��g��+2H
2O��l������H=-890.3kJ?mol
-1��
��H
2O��g��=H
2O��l����H=-44.0kJ?mol
-1��
���ø�˹���ɽ���+��-��-3���ٿɵã�CH
4��g��+H
2O��g��=CO��g��+3H
2��g��
��H=��-44.0kJ?mol
-1��+��-890.3kJ?mol
-1��-��-283.0kJ?mol
-1��-3����-285.8kJ?mol
-1��=+206.1 kJ?mol
-1��
�ʴ�Ϊ��CH
4��g��+H
2O��g��=CO��g��+3H
2��g����H=+206.1 kJ?mol
-1��
��2������ͼ���֪3minʱ��ƽ��ʱ�����Ũ��Ϊ0.1mol/L��������Ũ��Ϊ0.3mol/L����
CH
4��g��+H
2O��g��=CO��g��+3H
2��g��
��ʼ��mol/L����0.2 0.3 0 0
�仯��mol/L����0.1 0.1 0.1 0.3
ƽ�⣨mol/L����0.1 0.2 0.1 0.3
4minʱ�����Ũ��Ϊ0.09mol/L��Ũ�ȼ�С0.1-0.09=0.01
ˮ��Ũ��Ϊ0.19mol��Ũ�ȼ�С0.2-0.19=0.01��
CO��Ũ��Ϊ0.11��Ũ������0.11-0.1=0.01
������Ũ��Ϊ0.33��Ũ������0.33-0.3=0.03
Ũ�ȱ仯��֮��Ϊ1��1��1��3�����ڻ�ѧ������֮�ȣ��Ҽ����ˮ����Ũ�ȼ�С��һ����̼������Ũ������Ӧ��ƽ��������Ӧ�����ƶ������ݣ�1�������÷�Ӧ����Ӧ�����ȷ�Ӧ����3min�ı�����Ϊ�����¶ȣ�
�ʴ�Ϊ�����������¶ȣ�
��3���ٸ÷�Ӧ����ӦΪ���ȷ�Ӧ�������¶�ƽ��������Ӧ�����ƶ�������ĺ������ͣ����¶�t
1��t
2���ʴ�Ϊ������
��̼ˮ��
ֵԽ��ƽ��ʱ�����ת����Խ�ͣ�����Խ�ߣ���x
1��x
2���ʴ�Ϊ������
�۸÷�Ӧ����Ӧ�������������ķ�Ӧ������ѹǿƽ�����淴Ӧ�����ƶ���ƽ��ʱ����ĺ������ͣ���p
1��p
2���ʴ�Ϊ������
��4��������������ԭ��Ӧ�������������ŵ��������������ӣ������缫��ӦʽΪ��O
2+4e
-+2H
2O=4OH
-���ʴ�Ϊ��O
2+4e
-+2H
2O=4OH
-��
�ڲ��뷴Ӧ�������ڱ�״�������Ϊ8960mL�����ʵ���Ϊ
=0.4mol�����ݵ���ת���غ��֪�����ɶ�����̼Ϊ
=0.2mol��n��NaOH��=0.1L��3.0mol?L
-1=0.3mol��n��NaOH����n��CO
2��=0.3mol��0.2mol=3��2������1��1��2��1֮�䣬������̼��ء�̼����أ���̼��ء�̼����ص����ʵ����ֱ�Ϊxmol��ymol����x+y=0.2��2x+y=0.3�����x=0.1��y=0.1����Һ��̼���ˮ�⣬̼�������ˮ����ڵ��룬��Һ�ʼ��ԣ���c��OH
-����c��H
+����̼�����ˮ��̶ȴ���̼���������c��HCO
3-����c��CO
32-����������Ũ�����ˮ��̶Ȳ���̼���Ũ��ԭ�������������ӣ���c��K
+����c��HCO
3-����c��CO
32-����c��OH
-����c��H
+����
�ʴ�Ϊ��c��K
+����c��HCO
3-����c��CO
32-����c��OH
-����c��H
+����



 ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
