
����Ŀ����84����Һ������Чɱ�����HIN1�Ȳ�����ijͬѧ������һƿ����¶ʿ���ơ�84����Һ����������������Ϻ�����Һ��װ˵���õ�������Ϣ����84����Һ������25%NaClO��1000 mL���ܶ�1.192g/cm3��ϡ��100��������ȣ���ʹ�á������������Ϣ�����֪ʶ�ش��������⣺
��1��100gij84����Һ��3.55g���������������൱���ò�Ʒ����Ч�Ⱦ���3.55%������100gij84����Һ�к���___gNaClO��
��2��һƿ����¶ʿ���ơ�84����Һ����������տ�����CO2___L����״���������ʡ�
��3����ͬѧ���ġ���¶ʿ���ơ�84����Һ�����䷽������NaClO��������480 mL��25%NaClO������Һ������˵����ȷ����___�����ţ���
A.��ͼ��ʾ�������У��������Dz���Ҫ�ģ��������ֲ�������
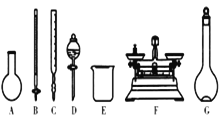
B.����ƿ������ˮϴ����Ӧ��ɲ���������Һ����
C.���ù������ƷNaClO�����ƿ��ܵ��½��ƫ��
D.��Ҫ������NaClO��������Ϊ143 g
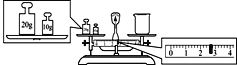
��4��ijͬѧ��������ƽ�����ձ�����������ƽƽ����״̬��ͼ����ͼ�п��Կ������ձ���ʵ������Ϊ___g��
��5������һ�����ʵ���Ũ�ȵ�������Һ�����в������������Ƶ�ϡ�������ʵ���Ũ��ƫ�͵���___(����ĸ)��
A��δ�ָ������¾ͽ���Һע������ƿ�����ж���
B������Ͳ��ȡŨ����ʱ���Ӱ�Һ��
C������ƿ������ˮϴ��δ����
D��δϴ���ձ��Ͳ�����
E������ʱ����Һ��
���𰸡�3.725g 89.6 AC 27.4 BDE
��������
(1). ����Ч�Ⱥ��������������������������������������䶨����:ÿ�˺��������������������൱�ڶ��ٿ�Cl2������������100gij84����Һ��3.55g���������������൱����������Ƶ����ʵ�����������ͬ����m=![]() �õ����ʵ�����
�õ����ʵ�����
��2��c=![]() ����Ũ��������ʵ���Ũ�ȣ����û�ѧ����ʽ�����������̼�����ʵ�����V=n��22.4�����������̼�������
����Ũ��������ʵ���Ũ�ȣ����û�ѧ����ʽ�����������̼�����ʵ�����V=n��22.4�����������̼�������
��3������������Һ�еIJ���������⣻��������һ�����ʵ���Ũ�ȵ���Һ�����Ʋ���ѡ��ʹ�õ�����������c=![]() ��ͨ���жϲ������������ʵ����ʵ���n����Һ���V��Ӱ����������
��ͨ���жϲ������������ʵ����ʵ���n����Һ���V��Ӱ����������
��4����ƽ��������ʱ��ѭ���������ԭ����ƽƽ��ԭ����������������=������������+����������
��5������һ�����ʵ���Ũ�ȵ���Һ�����Ʋ���ѡ��ʹ�õ�����������c=![]() ��ͨ���жϲ������������ʵ����ʵ���n����Һ���V��Ӱ����������
��ͨ���жϲ������������ʵ����ʵ���n����Һ���V��Ӱ����������
(1) ���ݷ����ɵã�100g����Һ�к��ȵ����ʵ���=![]() =
=![]() =0.05mol��������ԭ���غ㣬NaClO�����ʵ���Ϊ0.05mol����NaClO������Ϊ0.05mol��74.5= 3.725g��
=0.05mol��������ԭ���غ㣬NaClO�����ʵ���Ϊ0.05mol����NaClO������Ϊ0.05mol��74.5= 3.725g��
�ʴ�Ϊ3.725g��
(2) ��84����Һ����NaClO �����ʵ���Ũ��=![]() =
=![]() = 4.0mol/L�� n(NaClO)=1L��4.0 mol/L=4.0 mol�����ݷ�ӦCO2+NaClO+H2O�TNaHCO3+HClO������ҪCO2�����ʵ���Ϊn(NaClO)=4.0 mol������״����V(CO2)=4.0 mol��22.4 L/mol=89.6 L��
= 4.0mol/L�� n(NaClO)=1L��4.0 mol/L=4.0 mol�����ݷ�ӦCO2+NaClO+H2O�TNaHCO3+HClO������ҪCO2�����ʵ���Ϊn(NaClO)=4.0 mol������״����V(CO2)=4.0 mol��22.4 L/mol=89.6 L��
�ʴ�Ϊ��89.6��
��3��A������������ƽ����NaClO���壬�����ձ����ܽ�NaClO�����ò��������н������������������ƿ�ͽ�ͷ�ι������ݣ�ͼʾ��A. B. C. D����Ҫ�������貣�����ͽ�ͷ�ιܣ���A��ȷ��
B. ���ƹ�������Ҫ����ˮ�����Ծ�ϴ�Ӹɾ�������ƿ���غ�ɺ���ʹ�ã���B����
C. ����NaClO�����տ����е�H2O��CO2�����ʣ�������ƷNaClO���ܲ��ֱ��ʵ���NaClO���٣����Ƶ���Һ�����ʵ����ʵ�����С�����ƫ�ͣ���C��ȷ��
D. Ӧѡȡ500 mL������ƿ�������ƣ�Ȼ��ȡ��480 mL���ɣ�������ҪNaClO��������0.5 L��4.0 molL1��74.5 gmol1=149 g����D����
�ʴ�Ϊ��AC��
��4����ƽ���̵�����������������������������������������������30g�������������2.6g,���ձ�����������30.0g-2.6g=27.4g��
�ʴ�Ϊ��27.4g��
��5��������Һʱ��c=![]() ��������
��������
A��δ�ָ������¾ͽ���Һע������ƿ�����ж��ݣ���ʹVС��Ũ��ƫ��A����
B������Ͳ��ȡŨ����ʱ���Ӱ�Һ�棬��ʹnƫС��Ũ��ƫС����B��ȷ��
C������ƿ������ˮϴ��δ������滹Ҫ���ݣ�����Ӱ�죬��C����
D��δϴ���ձ��Ͳ���������ʹnƫС��Ũ��ƫС����D��ȷ��
E������ʱ����Һ�棬��ʹVƫ��Ũ��ƫС����E��ȷ��
�ʴ�ΪBDE��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͷ���ؾ��������Ŀ������ã���ϳ�·����ͼ��ʾ��
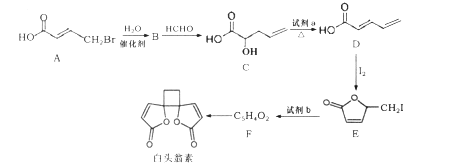
��֪��
��RCH2Br![]() RCH=CHR��
RCH=CHR��
��2RCH=CHR��![]()
������R��R�������⡢�����
(1)��ͷ���صķ���ʽΪ____��
(2)�Լ�aΪ______��E��F�ķ�Ӧ����Ϊ________��
(3)F�Ľṹ��ʽΪ_________��
(4)C�к��еĹ���������Ϊ________��
(5) A��B��Ӧ�Ļ�ѧ����ʽΪ_________��
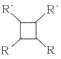
(6)F�����������ӳɵõ�G��G�ж���ͬ���칹�壬����������״��������____�֡�
(7)����ϩΪ��ʼԭ�ϣ�ѡ�ñ�Ҫ�����Լ��ϳ�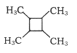 ��·��Ϊ____���ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ��������
��·��Ϊ____���ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��M��X��N��Z��Yԭ��������������Ķ����ڵ���������Ԫ�أ�����X��Zͬ���壬Y��Zͬ���ڣ�M��X��Y�Ȳ�ͬ�壬Ҳ��ͬ���ڡ�Xԭ�������������Ǻ�����Ӳ�����������Y������ϼ�������ͻ��ϼ۵Ĵ����͵���6��N�Ƕ���������Ԫ����ԭ�Ӱ뾶���ķǽ���Ԫ�ء�
(1)��д������Ԫ�ص�Ԫ�����ƣ�X________��M________��
(2) Y�����ڱ��е�λ��______________��д��Z���⻯��ĵ���ʽ_____________
(3) N��������������������Һ��Ӧ�Ļ�ѧ����ʽ_________________��
(4)Y��Z������������Ӧˮ���������ǿ��˳��________��________(�û�ѧʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵���У�����ȷ���ǣ� ��
A.0.1 mol N2��������2.8 g
B.���³�ѹ�£�22 g CO2��������ԭ����ԼΪ6.02��1023
C.2 L 0.1mol��L��1NaCl��Һ�У�c(Na��)��0.2mol��L��1
D.��״���£�11.2 L O2���е�ԭ����ĿԼΪ6.02��1023
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��һ�ֺ�������������ϵͳԭ��ʾ��ͼ������˵����ȷ����
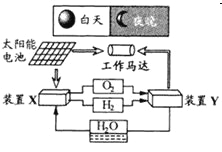
A. ��ϵͳ��ֻ����3����ʽ������ת��
B. װ��Y�и����ĵ缫��ӦʽΪ��![]()
C. װ��X��ʵ��ȼ�ϵ�ص�ȼ�Ϻ�����������
D. װ��X��Y�γɵ���ϵͳ��ʵ�����ʵ����ŷţ�����ʵ�ֻ�ѧ������ܼ����ȫת��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I�����Ǽ���ʵ���г��õ�������
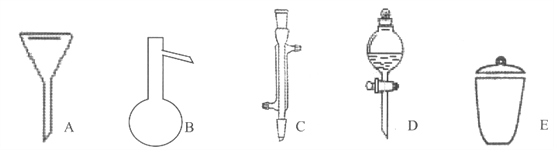
д����������������������ƣ�
A__________��B__________��C__________��D__________��E__________
IIʵ����Ҫ����100 mL 2 mol��L NaCl��Һ����ش��������⣺
��1�����ƹ�������Ҫʹ�õ���Ҫ�������������ձ�������������ͷ�ιܡ���ƽ����Ͳ��__________________��
��2����������ƽ��ȡ�Ȼ��ƹ��壬������Ϊ__________g��
��3��������Ҫ�����������ȷ˳����____________________������ţ���
�ٳ�ȡһ���������Ȼ��ƣ������ձ��У�����������ˮ�ܽ⣻
�ڼ�ˮ��Һ��������ƿ���̶�����1��2����ʱ�����ý�ͷ�ιܵμ�����ˮ����Һ����̶������У�
�۽���Һת�Ƶ�����ƿ�У�
�ܸǺ�ƿ�����������µߵ���ҡ�ȣ�
������������ˮϴ���ձ��ڱںͲ�����2��3�Σ�ϴ��Һת�Ƶ�����ƿ�С�
��4�����ʵ�������ȱ�ٲ���ݣ������������Һ�����ʵ���Ũ��__________ ���ƫ�ߡ���ƫ�͡�����Ӱ�족����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ����ɫ��ĩ��������NaCl��Na2CO3��Na2SO4�е�һ�ֻ�����ɣ�Ϊ������ɷֽ�������ʵ��(ÿ�������Լ�����������):
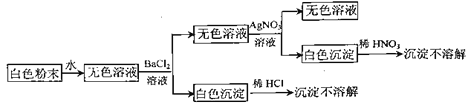
����˵���������ǣ� ��
A.��ĩ��һ������NaClB.��ĩ��һ������Na2SO4
C.��ĩ��һ������Na2CO3D.��ĩ�����Ǵ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
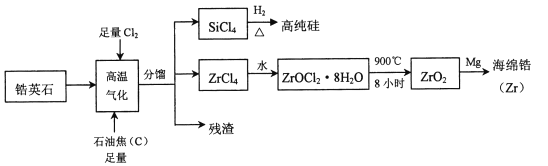
����Ŀ���(![]() )���ִ���ҵ����Ҫ����ԭ�ϣ��������õĿ����ԣ���ʴ���ܳ����ѡ����Ӣʯ(��Ҫ�ɷ���
)���ִ���ҵ����Ҫ����ԭ�ϣ��������õĿ����ԣ���ʴ���ܳ����ѡ����Ӣʯ(��Ҫ�ɷ���![]() ������������
������������![]() ������)Ϊԭ������ﯼ��仯�����������ͼ��ʾ
������)Ϊԭ������ﯼ��仯�����������ͼ��ʾ
(1)д��![]() �ĵ���ʽ____________________��
�ĵ���ʽ____________________��
(2)�������������У���������ʧ�����ַ����õ���![]() ����ͨ�����ɴ�
����ͨ�����ɴ�![]() �����ķ�Ӧ�õ���
�����ķ�Ӧ�õ���![]() �������û�ѧ����ʽ������ԭ��_________________________��
�������û�ѧ����ʽ������ԭ��_________________________��
(3)����������Ĺ��������̼�⣬����![]() ��
��![]() ����ˮ��ȡ���Ȼ�����Һ�����ˣ��������з����̼��
����ˮ��ȡ���Ȼ�����Һ�����ˣ��������з����̼��![]() ���ֹ���ķ�����____________________��
���ֹ���ķ�����____________________��
(4)д������������![]() ��ˮ��Ӧ�Ļ�ѧ����ʽ��____________________________��
��ˮ��Ӧ�Ļ�ѧ����ʽ��____________________________��
(5)��֪�����(![]() )��һ��������������������ƹ����ڿ��γ����Σ���д����ѧ����ʽ_________________________________________��
)��һ��������������������ƹ����ڿ��γ����Σ���д����ѧ����ʽ_________________________________________��
(6)��ҵ�Ͽ��üػ�ԭ![]() ʱ�Ƶý���
ʱ�Ƶý���![]() ��
��![]() ����ԭʱ���ɵļ��ε����ʵ���Ϊ_________________��
����ԭʱ���ɵļ��ε����ʵ���Ϊ_________________��
(7)��֪![]() ��
��![]() ������˵����ȷ����__________
������˵����ȷ����__________
A��![]() ���������Һ��
���������Һ��![]() ��
��![]() ֮�;�Ϊ14
֮�;�Ϊ14
B��������ζ�ijŨ�ȵ�![]() ��Һ���ζ�������
��Һ���ζ�������![]() ������
������
C��![]() ��Һ����μ���������Һ���μӹ�����
��Һ����μ���������Һ���μӹ�����![]() ��С
��С
D��ij�¶���![]() ��������Һ��
��������Һ��![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������500 mL0.5mol/LNaOH��Һ���Իش��������⣺
��1����Ҫ��ȡNaOH���������Ϊ___��
��2�����Ʒ������������������裬���ں��������Ϻ��ʵ���������
����ʢ��NaOH���ձ��м���100mL����ˮʹ���ܽ⣬����ȴ�����¡�
�ڽ�NaOH��Һ��___ע��___�С�
�����ձ��м�������������ˮ��С��ϴ��___2��3�β���ÿ�ε�ϴ��Һ��ת����___��
�ܼ�����___�м�����ˮ��Һ��ӽ��̶���1��2cm��
�ݸ���___�μ�����ˮ���̶��ߣ��Ǻ�ҡ�ȡ�
��3�����²�����ʹʵ������NaOH��Һ��Ũ��ƫ�͵���___��
A������ʱ���ӿ̶��� B���ܽ����ձ�δ��ϴ��
C�������õ��ձ������� D������ƿ��ԭ��������������ˮ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com