
| Fe |
| Fe |
| Fe |
| Fe |


 ��ٽ������½������������ϵ�д�
��ٽ������½������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��������Ϫһ�и߶���ѧ�����п������ƻ�ѧ�Ծ����������� ���ͣ�ʵ����
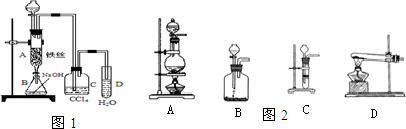
��15�֣���1��ij��ѧ����С������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A��A�¶˻����رգ��С�
��д��A���л���Ӧ�Ļ�ѧ����ʽ ��
����֪�����л���Ӧ�Ƿ��ȷ�Ӧ���۲쵽A�е������ǣ�
��_____ _________��
�� ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������Ŀ���� ��д���йصĻ�ѧ����ʽ ��
��C��ʢ��CCl4�������� ��
����֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�е���AgNO3��Һ������������ɫ����������֤������һ����֤�ķ��������Թ�D�м���______ _____��������______________________��
��2����Ȳ��ʵ�����Ʒ�
�ٷ�Ӧԭ��_____ ____________��
��ѡ����ʵ���ȡʵ��װ��___ ___��
��ʵ���г��ñ���ʳ��ˮ����ˮ��Ŀ����______ __________��
�ܴ�������Ȳ��������ɫ��ζ�����壬�õ�ʯ��ˮ��Ӧ��ȡ����Ȳ��������H2S��PH3���ж����ζ��������____ _______��Һ��ȥ�������塣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014������ʡ�ɽ�ظ�����ѧ������������⻯ѧ�Ծ��������棩 ���ͣ������
��1��ij��ѧ����С���ú���Ϊԭ����ȡ������ˮ�����������Ȼ�̼�ڵ�ˮ����ȡ�ⵥ�ʣ����÷�Һ©������������Һ����ʵ������ɷֽ�Ϊ���¼�����
A����ʢ����Һ�ķ�Һ©����������̨����Ȧ�У�
B����50mL��ˮ��15mL���Ȼ�̼�����Һ©���У����Ǻò�������
C�������Һ©���������Ͽڲ������Ƿ�©Һ��
D����ת©��������������ʱ�����������������رջ�����
E���ſ����������ձ�������Һ��
F���ӷ�Һ©���Ͽڵ����ϲ�ˮ��Һ��
G����©���ϿڵIJ�������ʹ���ϵİ��ۣ���С�ף���©�����ϵ�С�ף�
H�����á��ֲ㡣�ʹ�ʵ�飬���������գ�
��ȷ���������˳���ǣ� ��
��2����MnSO4������Ļ����Һ�����K2S2O8����������أ�����Һ�ᷢ���·�Ӧ��Mn2++S2O82‾+H2O��MnO4‾+SO42‾+H+
�÷�Ӧ�����ڼ���Mn2+�Ĵ��ڡ������������� ��
���÷�Ӧ���õ������̸�Ϊ�Ȼ��̣������������Ĺ�����ط�Ӧʱ�����и�����ء�����ء����������⣬�����������ﻹ�� ��
��3����NaBiO3���壨��ɫ�ܣ����뵽MnSO4��H2SO4�Ļ����Һ����ȣ������ܽ��Ϊ������Һ���������·�Ӧ�� NaBiO3+ MnSO4+ H2SO4�� Na2SO4+ Bi2(SO4)3+ NaMnO4+ H2O
����ƽ������Ӧ�Ļ�ѧ����ʽ�����������ת�Ƶķ������Ŀ��
��������Ӧ�л�ԭ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
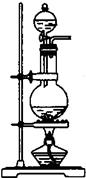
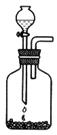
��1��ij��ѧ����С������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A��A�¶˻����رգ��С�
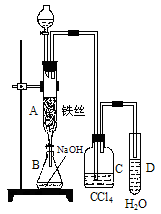
��д��A���л���Ӧ�Ļ�ѧ����ʽ
___________________________________________________
����֪�����л���Ӧ�Ƿ��ȷ�Ӧ���۲쵽A�е�������
_____________________��______________________��
�� ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������Ŀ����_______________________________________________��д���йصĻ�ѧ����ʽ____________________________________________��
��C��ʢ��CCl4��������______________________________________��
����֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�е���AgNO3��Һ������������ɫ����������֤������һ����֤�ķ��������Թ�D�м���__________________��������___________________________________________��
��2����Ȳ��ʵ�����Ʒ�
�ٷ�Ӧԭ��___________________________________________________��
��ѡ����ʵ���ȡʵ��װ��_______��

 |  | ||
����
������A������������ �������� B������������������������ C�������� ������������ D
��ʵ���г��ñ���ʳ��ˮ����ˮ��Ŀ����___________________________________��
�ܴ�������Ȳ��������ɫ��ζ�����壬�õ�ʯ��ˮ��Ӧ��ȡ����Ȳ��������H2S��PH3���ж����ζ��������________________��Һ��ȥ�������塣
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com