
����Ŀ����������500 mL 0.040 mol��L-1��K2Cr2O7��Һ��
��1������������У�������ƽ��ҩ�ס��ձ���________��________��________�����ں�������д��ȱ���������ƣ�
��2������Һ�����ƹ����У������»���ʵ�鲽�裬��ȷ�IJ���˳���ǣ���д��������Ĵ��ţ�ÿ����������ֻ��һ�Σ�________��
�ٵߵ�ҡ�� �ڶ��� ��ϴ�� ���ܽ� ��ת�� ����
��3����������ƽ��ȡK2Cr2O7���������Ϊ________ g��
��4�����в���ʹ���ʵ����ƫС����________������ţ���
A����ˮ����ʱ���ӿ̶��� B��ת��ǰ������ƿ�к�����������ˮδ����
C��δϴ���ձ��ڱںͲ����� D��ҡ�Ⱥ��ְ�Һ����ڿ̶����ּ�ˮ����
��5������ʱ�������С�ļ�ˮ�����˿̶��ߣ������ķ�����________ ��
���𰸡������� 500 mL����ƿ ��ͷ�ι� �ޢܢݢۢڢ� 5.9 C D ��������
��������
��1���������ƵIJ���������������Ҫ��������
��2�����Ʋ����Ǽ��㡢�������ܽ⡢ת�ơ�ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��
��3������n = cV��m = nM�����500mL 0.040 mol��L-1��K2Cr2O7��Һ�к�������K2Cr2O7��������
��4������ʵ�������c = ![]() ��Ӱ�������������
��Ӱ�������������
��5�����ƹ����еIJ���ʧ���ܲ��ȾͲ��ȣ����ܲ��Ⱦ����������ƣ�
��1�����������м��㡢�������ܽ⡢ת�ơ�ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ�(������Ͳ��ȡˮ�����ձ�)�����ò��������裬�����ܽ⡣��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ���ձ���������23�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���12cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȡ���������������������ƽ��ҩ�ס��ձ����⣬��ȱ��������500mL����ƿ�ͽ�ͷ�ιܣ�
�ʴ�Ϊ����������500mL����ƿ�ͽ�ͷ�ιܣ�
��2�����������������������Ժ�����һ��Ũ�ȵ���Һ������������Ϊ�������ܽ⡢ת�ơ�ϴ�ӡ����ݡ�ҡ�ȵȹ��̣�������ȷ��˳��Ϊ���ޢܢݢۢڢ���
�ʴ�Ϊ���ޢܢݢۢڢ���
��3����Һ������K2Cr2O7�����ʵ���Ϊ��n = cV = 0.040 mol��L-1![]() 0.5 L = 0.020 mol����K2Cr2O7��Ħ������Ϊ��M = ��39
0.5 L = 0.020 mol����K2Cr2O7��Ħ������Ϊ��M = ��39![]() 2+52
2+52![]() 2+16
2+16![]() 7 ��g/mol= 294 g/mol���ٸ���m = nM = 0. 020 mol
7 ��g/mol= 294 g/mol���ٸ���m = nM = 0. 020 mol![]() 294 g/mol = 5.88 g��ʵ�ʲ���ʱ������ƽֻ�ܾ�ȷ��С�����һλ����˰���������ԭ����������ƽ����������Ϊ5.9 g,
294 g/mol = 5.88 g��ʵ�ʲ���ʱ������ƽֻ�ܾ�ȷ��С�����һλ����˰���������ԭ����������ƽ����������Ϊ5.9 g,
�ʴ�Ϊ��5.9 ��
��4��A.����ʱ���ӿ̶��ߣ��������Ƶ���Һ���ƫС�������Ƶ���ҺŨ��ƫ�ߣ����������⣬��A�������
B.ת��ǰ������ƿ�к�����������ˮδ�������Բ��Խ����Ӱ�죬��Ϊת���Ժ�ҲҪ��ˮ���̶���1-2cm�ٶ��ݣ�ֻҪ���ݲ�������ԭ�е���������ˮ��Ũ����Ӱ�죬��B�����������
C. δϴ���ձ��ڱںͲ���������ʹ�����������٣��������ʵ������٣���ҺŨ��ƫ������C�����������
D. ҡ�Ⱥ��ְ�Һ����ڿ̶����ּ�ˮ���ϣ��൱��ϡ����ԭ��Һ�����ʹ��ҺŨ��ƫ�ͣ���D�����������
�ʴ�Ϊ��CD��
��5�������Ǽ�ˮ�����˿̶��ߣ��������ȵģ���Ӧ�������ƣ�
�ʴ�Ϊ���������ƣ�


 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����л�ѧ�����ʾ��ȷ����
A. ���������ĵ��뷽��ʽ��Fe2��SO4��3 ��2Fe3+��3SO42��
B. H2SO4�ĵ��뷽��ʽ��H2SO4��H2+ + SO42-
C. ������Ϊ6��������Ϊ7������76C
D. �����ӵĽṹʾ��ͼ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ��Ԥ�ԣ�ȼ�ϵ�ؽ���21���ͻ�õ�������Ҫ;���������Ѽƻ����״�ȼ�ϵ�����ھ���Ŀ�ġ�һ�ּ״�ȼ�ϵ���Dz��ò���̼������Ϊ�缫��������ϡ������Һ��ֱ�Ӽ��봿����ļ״���ͬʱ��һ���缫ͨ��������ش��������⣺
��1�����ֵ�طŵ�ʱ�����Ļ�ѧ��Ӧ����ʽ________________��
��2���˵�ص����������ĵ缫��Ӧʽ��_______________�����������ĵ缫��Ӧʽ��_________��
��3�����Һ�е�![]() ������__________���ƶ��������·�ͷŵ��ӵĵ缫��__________��
������__________���ƶ��������·�ͷŵ��ӵĵ缫��__________��
��4������ֱ��ȼ��ȼ�ϲ���������ʹ��ȼ�ϵ���������ŵ㣬������Ҫ�����㣺������ȼ�ϵ�ص�����ת��Ч�ʸߣ������____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ��ȥ�����е�Ca2+��Mg2+��SO42���Լ���ɳ�����ʣ�ijͬѧ�����һ���Ʊ����ε�ʵ�鷽�����������£����ڳ������Լ��Թ�������
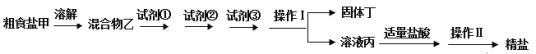
��1����������Ҫ�õ��IJ���������________���������������________��
��2���Լ��١��ڡ�����ʵ���������ʣ�����Na2CO3��Һ��BaCl2��Һ��NaOH��Һ�����������ʵļ���˳������ж������������ѡ������ȷ����________��
A������Na2CO3��Һ��BaCl2��Һ��NaOH��Һ
B��BaCl2��Һ������Na2CO3��Һ��NaOH��Һ
C��NaOH��Һ��BaCl2��Һ������Na2CO3��Һ
D������Na2CO3��Һ��NaOH��Һ��BaCl2��Һ
��3�����嶡�ǻ�����������ɳ��CaCO3��BaSO4�⣬������________���ѧʽ����
��4���ڻ�������зֱ�����Լ��١��ڡ��۵Ĺ����У��жϵμ�BaCl2��Һ�ѹ����ķ����ǣ�����BaCl2��Һ���ã����ϲ���Һ�У�________��
��5�������������������pHֵ�ٽ��в�������ʵ��������Ӱ�죬��ԭ����______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������һ�����ʵ���Ũ�ȵ�KOH��Һʱ������Ũ��ƫ�͵�ԭ�������(����)
A. �ó�����������KOH��ʱ�����
B. ����ǰ��������ƿ�м�����������ˮ
C. ����ƿʢ��KOH��Һ��ʹ��ǰδϴ��
D. �ܽ�����ת�Ƶ�����ƿ��Ȼ�����������ˮ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵���д������
A. Ϊ�˳�ȥMgCl2������Һ�е�Fe3+�����ڼ��������¼���NaOH��Һ
B. ��K2Cr2O7��Һ�м�������ŨNaOH��Һ����Һ�ɳ�ɫ��ɻ�ɫ
C. ����KI��H2SO4�Ļ����Һ����һ��ʱ����������Һ����Һ����
D. �μ�أ����϶�NaCl��Na2CO3���м���������ʯ�ࣨCaSO4�����Խ��������ļ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E��FΪǰ������ԭ�������������������Ԫ�أ�A��C��Dԭ�Ӿ�������δ�ɶԵ��ӣ�A��B��Cͬ���ڣ�A��D��B��F�ֱ�ͬ���壬E���������������Ľ�������ش��������⣺
��1��E�Ƚ��ȶ������Ӻ�������Ų�ʽ_____________________________��
��2��A��B��C�ĵ�һ��������С�����˳��Ϊ_________________![]() ��Ԫ�ط��ű�ʾ
��Ԫ�ط��ű�ʾ![]() ��B��C�ļ��⻯�����������������ʵĻ�ѧʽ___________��
��B��C�ļ��⻯�����������������ʵĻ�ѧʽ___________��
��3��C��D�γɵ����ʵľ���������____________��IT��ҵ�иþ�����������____________��
��4����A��B��C����Ԫ���е�һ�ֻ�����Ԫ���γɵķ����У��еĻ�Ϊ�ȵ����壬д������һ��ȵ�����Ļ�ѧʽ��______![]() ��д����Ӧ�Ľṹʽ_______________��
��д����Ӧ�Ľṹʽ_______________��
��5��B�ĵ��ʾ�����![]() �����ƣ���һ�������к�B��ԭ�Ӹ���Ϊ____
�����ƣ���һ�������к�B��ԭ�Ӹ���Ϊ____![]() �����γɵķ��ӿռ乹����_______��
�����γɵķ��ӿռ乹����_______��
��6������˪����һ�ֺ�C��F�Ļ��������ӽṹ��ͼ1��ʾ���û�����ķ���ʽΪF4C6��Fԭ�Ӳ�ȡ______�ӻ���C��D��E��ɵĻ�����ľ�����ͼ2���侧������Ϊa pm�������ܶ�Ϊ___________________g/cm3���г�ʽ�Ӽ��ɣ�����٤������ΪNAmol-1����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ��̽����������ˮ�Ļ�ԭ�ԣ�ij��ȤС��ͬѧ���������̽�����
I.̽�������Ļ�ԭ��
����ȤС��ͬѧ��������װ��(�г֣�����������)̽�������백���ķ�Ӧ������A��F�ֱ�Ϊ�����Ͱ����ķ���װ����BΪ��������������백����Ӧ��װ�á�
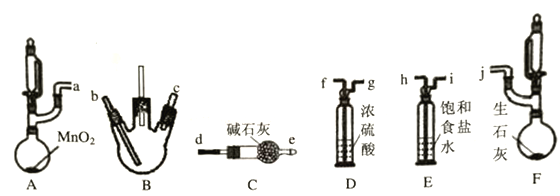
��ش�����������
��1������װ�ýӿڵ�����˳��Ϊa��h��i��f��g��___��____��___��____��j������װ��D��������____________��
(2)������������װ��B�г��ֵ�����Ϊ____________��
II.̽����ˮ�Ļ�ԭ��
����ȤС��ͬѧ̽����ͬ�����¸��������Һ�백ˮ�ķ�Ӧ��ʵ������:
ʵ�� | ���� | ���� |
�� | ȡ2mL.0.01mol/LKMnO4��Һ���Թ��У������¿���1mLŨ��ˮ�������ι�����ˮ��������Ƥ����ס�� | �����غ�ɫ����(MnO2),Լ10min����Һ�Ϻ�ɫ��dz |
�� | ȡ2mL0.01mol/LKMnO4��Һ���Թ��У������¿���1mLŨ��ˮ�������ι�1:5�����ᣬ������Ƥ����ס�� | �����غ�ɫ����(MnO2),��Һ�Ϻ�ɫ���̱�dz��Լ2min����Һ�Ϻ�ɫ��ȫ��ȥ |
�� | ȡ2mL0.1mol/LKMnO4��Һ���Թ��У������¿���ImLŨ��ˮ�������ι�����ˮ��������Ƥ����ס�� | �����غ�ɫ����(MnO2),Լ10min����Һ�Ϻ�ɫ��dz |
�� | ȡ2mL0.1mol/LKMnO4��Һ���Թ��У������¿���1mLŨ��ˮ�����˰�ι�1:5�����ᣬ������Ƥ����ס�� | �����غ�ɫ����(MnO2)����Һ�Ϻ�ɫ���̱�dz��Լ5min����Һ�Ϻ�ɫ��ȫ��ȥ |
��3��ʵ��������������ΪN2��д���÷�Ӧ�����ӷ���ʽ:_________��
��4��ʵ���٢�˵��________________��
��5��ʵ������ʵ������Ӧ����_____(����������������)��ԭ����_________��
��6��1:5��������Һ(�ܶ�Ϊ��2g��cm-3)��������������Ϊ98%��Ũ����(�ܶ�Ϊ��1g��cm-3)��
����ˮ�������1:5��ɣ����1:5��������Һ�����ʵ���Ũ��Ϊ_____mol/L��(�ú���1����2��ʽ�ӱ�ʾ)
��7����ʵ��I��II�ɵó��Ľ�����____________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com