
����Ŀ��.ij��ȤС�����10.0 g�������ޣ���90%��Al��������������Fe��Mg�����ʣ��Ʊ�����[KAl(SO4)2��12H2O]��ʵ��������ͼ��

��1���Լ���Ӧѡ��________������ĸ����
a.���� b.H2SO4��Һ c.��ˮ d.NaOH��Һ
��2���������ܽ��������Ҫ��Ӧ�Ļ�ѧ����ʽΪ__________________________��
��3������ҺC�еõ�������ʵ�����Ϊ________��________�����ˣ�����ͼ��ʾװ�ý��иò��������е�һ����Ҫ������______________��
���𰸡�d 2Al��2NaOH��2H2O=2NaAlO2��3H2�� ����Ũ�� ��ȴ�ᾧ ��������ʹ������
��������
Al����ǿ�Ӧ����Fe��Mg����Ӧ�����Լ���ΪNaOH��Һ����ҺAΪƫ�����ƺ�����δ��Ӧ��NaOH������AΪFe��Mg���壻��ƫ��������Һ��ͨ������Ķ�����̼��������������������̼��������Һ�������BΪ������������ҺBΪ̼��������Һ�����������������������صĻ��Һ������ҺCΪ����������Һ��
(1)������֪���Լ���ΪNaOH��Һ����Ϊd��
(2)�������е�Al��NaOH��Ӧ����ƫ�����ƺ�����������ʽΪ2Al��2NaOH��2H2O=2NaAlO2��3H2����
(3)��ҺCΪ����������Һ���ɲ�������Ũ�������½ᾧ�����ˡ�ϴ�ӵķ����õ�������������Һʱ��ʹ��������ʹ��������


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)���������ڱ�������Ԫ�ذ��� ______________________(��Ԫ������)����Ԫ�أ�����Se�Ļ�̬ԭ�ӵĵ����Ų�ʽΪ______________________��Ԫ��X��Seͬ���ڣ�XԪ��ԭ�Ӻ���δ�ɶԵ�������࣬XΪ______________________(��Ԫ�ط���)��
(2)����ͬ���ڵ�����Ԫ���У���һ�������д�С��˳��______________________��
(3)�����ŷŵij�����Ҫ�ɷ�Ϊ3��MBT(3������2����ϩ���ṹ��ͼ)��1mol3��MBT�к���������ĿΪ____________(NAΪ����ӵ�������ֵ)���е㣺3��MBT____________(CH3)2C==CHCH2OH(������������������=��)����Ҫԭ����________________________��

(4)S��+4��+6���ּ�̬�������
�����й�����̬SO3��SO2��˵����ȷ����__________(�����)��
A.����ԭ�ӵļ۲���Ӷ��������
B.���Ǽ��Է���
C.����ԭ�ӵĺ˶Ե�����Ŀ�����
D.�����м��Լ�
��SO3���ӵĿռ乹��Ϊ__________�����以Ϊ�ȵ������������Ϊ__________(��һ��)��
(5)����Po����__________���γɵľ��壻����֪Po��Ħ������ΪMg��mol-1��ԭ�Ӱ뾶Ϊrpm�������ӵ�������ֵΪNA�����Ǿ�����ܶȵı���ʽΪ____________________g��cm-3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ұ���������ķ���������Ϊԭ����ȡ��ϸ![]() -�����������ܽ��ͻ�����Ⱦ�ֿ��������Դ������������֪���ҵ���Ҫ�ɷ�ΪA12O3(����������SiO2��FeO��Fe2O3)�����Ʊ��������£�
-�����������ܽ��ͻ�����Ⱦ�ֿ��������Դ������������֪���ҵ���Ҫ�ɷ�ΪA12O3(����������SiO2��FeO��Fe2O3)�����Ʊ��������£�
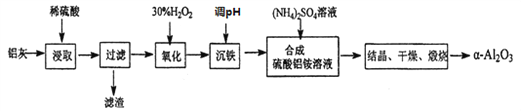
��1������ͼ������������NaOH�����Ʊ������ƣ��ɲ��õ�װ��Ϊ____(��ѡ����)��

��2�������м���H2O2 �����������ԭ����_______________________________��
��3��ͨ��������Һ��pH�������������õ�Fe(OH)3����֪��
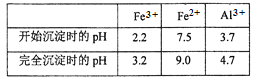
Ϊ��֤��Ʒ�Ĵ��ȣ�����ѡ�����������е�_______������ҺpH(����ĸ)������pH�ķ�ΧΪ___________________________��
a��A12O3 b��NaOH c��Al(OH) 3 d��Na2CO3
��4������������茶��壬��������Ҫ��ӦΪ��
4[NH4 Al(SO4)2��12H2O] ![]() 2Al2O3+2NH3��+N2��+5SO3��+3SO2��+53H2O��
2Al2O3+2NH3��+N2��+5SO3��+3SO2��+53H2O��
������������ͨ����ͼ��ʾ��װ����
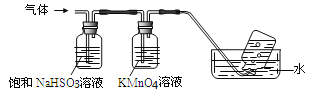
������ƿ���ռ�����������_____________________(�ѧʽ)��
��װ��KMnO4��Һϴ��ƿ��������__________________________________��
��ѡ��һ�ֳ��û�ѧ�Լ���ϡ�������������泥����Լ���______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�ʵ��IJ�����ȷ����
ʵ��Ŀ�� | ʵ����� | |
A | ����Ũ��Ϊ0.010 | ��������ƽ��ȡKMnO4����0.158 g������100 mL����ƿ�У���ˮ�ܽⲢϡ�����̶� |
B | Ũ������MnO2��Ӧ�Ʊ�����Cl2 | ���������ͨ��Ũ���ᣬ��ͨ������ʳ��ˮ |
C | ����ϡ���� | �Ƚ�Ũ��������ձ��У���������ˮ |
D | ��ˮ���ռ�KMnO4�ֽ������O2 | ���Ƴ����ܣ���Ϩ��ƾ��� |
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ӹ�ҵ����30%��FeCl3��Һ��ʴ���ھ�Ե���ϵ�ͭ��������ӡˢ��·�塣
(1)д��FeCl3��Һ�����ͭ������Ӧ�Ļ�ѧ����ʽ:______________________��
(2)ij����ʦΪ�˴�ʹ�ù��ĸ�ʴ��Һ����Ҫ��CuCl2��FeCl3��Һ�ȣ��л���ͭ�������»�ô�����FeCl3��Һ����������ͼ��ʾ����:����֪���ӹ�����ΪFe���ӹ�����Ϊϡ���ͨ����ΪCl2��
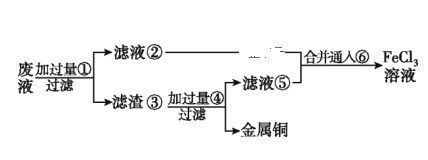
������ʵ��������������Ҫ����Ϊ___________________���ѧʽ����д�����ڢ��ĺϲ�Һ��ͨ���������ӷ���ʽ:______________________________��
�����Ʋ�����FeCl2��Һʱ���������м����Ŀ����_______________________________��
��Ҫ֤��FeCl3��Һ�Ƿ���Fe2+����ѡ������ѡ���е�______________����֪����������ʺ�������ɫ��������
A������ K3[Fe(CN)6]��Һ ��B������NaOH��Һ C������KSCN��Һ
(3)����1.2 L��3 mol FeCl2��������Һǡ����0.6 mol HIO3��ȫ��Ӧ����ԭ����Ϊ_______���ѧʽ����֪��������������Һ���������÷�Ӧ���ӷ���ʽΪ___________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Է�ӦA��B��AB��˵�������°�����������з�Ӧ��
��20 mL��Һ�к�A��B��0.01 mol
��50 mL��Һ�к�A��B��0.05 mol
��0.1 mol��L��1��A��B��Һ��10 mL
��0.5 mol��L��1��A��B��Һ��50 mL
���߷�Ӧ���ʵĴ�С��ϵ��
A.�ڣ��٣��ܣ���B.�ܣ��ۣ��ڣ���
C.�٣��ڣ��ܣ���D.�٣��ڣ��ۣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Ʒ����ظ����ƣ� Na2Cr2O72H2O )����Ҫ�Ļ�������ԭ�ϣ�������ˮ����ǿ������ ��������2012 ���й���ѧԺ�о����������Ը��ᱵΪ�м��壬��������Ʊ��ߴ����ظ����Ƶķ������÷�����ת�������ºͣ���Ⱦ���ŷ��٣�����������ʾ��
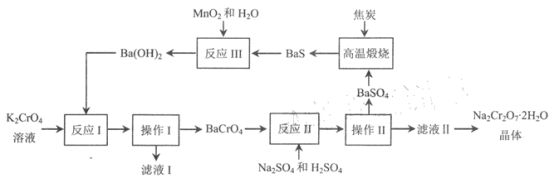
�ش��������⣺
(1)����I�Ͳ���II�����ƾ��� ____________, ��Һ I�����ʵ���Ҫ�ɷֵĻ�ѧʽΪ_____________��
(2)��ӦI��II��III������������ԭ��Ӧ����____________________��
(3)��������ʱ����������̿��������Ӧ�Ļ�ѧ����ʽΪ ________________��
(4)��ƽ��Ƕȷ�������Ӧ II ��H2SO4���� pH ��ԭ����_______________�������ӷ���ʽ��ʾ���� ��Ӧ II ������ HCl ���H2SO4 ��ԭ����_________________��
(5)��Cr2O72- �ķ�ˮ�辭��ѧ������ʹ��Ũ�Ƚ������Ϲ����йر������ŷš�ͨ���������Է�ˮ���ȼ��������̷�(FeSO47H2O ) ����Cr2O72- ��ԭ��Cr3+��������Ӧ�����ӷ���ʽΪ___________�� �ټ��������ʯ��ˮ��ʹ Cr3+ת��ΪCr(OH)3�������ø÷������� c(Cr2O72-) =1.5��10-3 mol��L-1�ķ�ˮ 10 m3��������Ҫ�̷�______kg��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼʾ�������������Ӧʵ�����
A.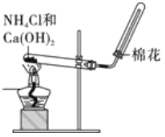 ʵ������ȡ���ռ�NH3
ʵ������ȡ���ռ�NH3
B.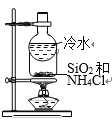 ����SiO2��NH4Cl
����SiO2��NH4Cl
C. ������ᾧ�����Ƿ��нᾧˮ
������ᾧ�����Ƿ��нᾧˮ
D.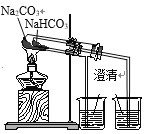 ��֤���ȶ��ԣ�Na2CO3>NaHCO3
��֤���ȶ��ԣ�Na2CO3>NaHCO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ڷ��õ�FeSO4��Һ�ױ����������ʣ�ij��ȤС�����������ʵ�飺
(1)���ʵ�����FeSO4��Һ�ı��ʳ̶�
ʵ�鷽�� | ʵ������ | ʵ����� | |
����1 | ȡ�����Һ���Թ��У������еμ�KSCN��Һ | ________ | FeSO4��Һ���ֱ��� |
����2 | _____ | _________ | |
�� ������������������
�� ��Ҫʹ���ֱ��ʵ�FeSO4��ԭ��������__________��(д���ӷ�Ӧ����ʽ)
(2)�������ֱ�����FeSO4��Һ�Ʊ�Fe2O3
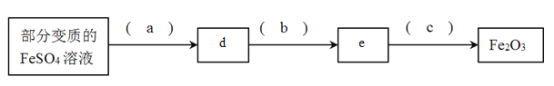
����д���и��գ�a._____b._______c.________d._____e.______
����100mL�ñ�����Һ�Ƶ�1.6gFe2O3�������ǰFeSO4��Һ��Ũ��Ϊ__________��
(3)FeSO4��������������ʹ��ʱ������ά����Cͬ����ͬѧ�ײ²�ά����C�ɽ�Fe3+ת��ΪFe2+���������������ա�Ϊ����֤��һ���룬���������ʵ�飺
ʵ�鷽�� | ʵ������ |
ȡ���� Fe2(SO4)3��Һ���Թ��У�����ά����CƬ�����ܽ�μ����Ը��������Һ�� | ��ɫ��ȥ |
������ʵ���ܷ�ó���ά����C�ɽ�Fe3+ת��ΪFe2+���Ľ��ۣ���˵������_______��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com