
����Ŀ���軯��(NaCN)��һ����Ҫ�Ļ���ԭ�ϣ��ڹ�ҵ����������;��NaCN�о綾�������ˮ�Ĺ�ҵ����������ͼ��ʾ��

��֪��Ka(HCN)< Ka(CH3COOH)��[Ag(CN)2]-(aq)![]() Ag+(aq)+2CN-(aq)��
Ag+(aq)+2CN-(aq)��
��1���軯����ˮ������綾�����ᣬд���÷�Ӧ�����ӷ���ʽ��_____________________________��
�����ʵ���Ũ����ͬʱ��CH3COOH��ˮ�������c(H+)_____________(����ڡ�����С�ڡ����ڡ�)HCN��ˮ�������c(H+)��
��2����������ͨ��ˮ������Ŀ����____________________________________________________��
��3���������У�C1O2�ɽ�CN-�������Ҳ������������壬����Ӧ������ת���� 6.02��1023������ʱ�����ɱ�״����������������_____________L��
��4���������У�Ҳ����H2O2����C1O2����Ӧ������һ����ʹ��ɫʯ����ֽ�����������һ�ֳ�������ʽ�Σ�д���÷�Ӧ�����ӷ���ʽ��_______________________________________________________��
��5����Ƴ������ʱ��Ҫ���ͶƲ�����ij������ʣ�ʹ�Ʋ�������ܡ������Һʹ��Na[Ag(CN)2]��Һ����������ӦʽΪ__________________________________________������ҵ�����ʹ��������(CN-)��ԭ��_______________________________________��
���𰸡� CN-+H2O![]() HCN+OH- С�� ��ʹHCN�ӷ����������� 6.72 CN-+H2O2+H2O=HCO3-+NH3�� [Ag(CN)2]-+e-=Ag+2CN- Ag+��CN-���Խ�������ȶ���[Ag(CN)2]-���Կ���������Ũ�ȣ�ʹ�Ʋ����ܣ������������𰸣�
HCN+OH- С�� ��ʹHCN�ӷ����������� 6.72 CN-+H2O2+H2O=HCO3-+NH3�� [Ag(CN)2]-+e-=Ag+2CN- Ag+��CN-���Խ�������ȶ���[Ag(CN)2]-���Կ���������Ũ�ȣ�ʹ�Ʋ����ܣ������������𰸣�
��������(1)�軯������ˮ������軯�⣬����ʽΪCN-+H2OHCN+OH-�����������Ա�HCNǿ����Һ��H+Ũ��Խ��ˮ�ĵ�������Խ���������ʵ���Ũ����ͬʱ��CH3COOH��ˮ�������c(H+)С��HCN��ˮ�������c(H+)��
(2)��������ͨˮ������Ŀ���Ǵ�ʹHCN�ӷ���������������
(3)CN-��C1O2�������������������ǵ����Ͷ�����̼����2CN-+2C1O2=N2��+2CO2��+2Cl-����Ӧ��ÿĦC1O2�μӷ�Ӧת��5mol���ӣ�����Ӧ������ת�� 6.02��1023��1mol�����ǣ��μӷ�Ӧ��C1O2Ϊ0.2mol������������������ʵ���Ϊ0.3mol����״����������������0.3mol��22.4L/mol=6.72L��
(4)CN-��˫��ˮ��Ӧ����һ����ʽ��Ϊ̼�����ƣ�ͬʱ����һ����ʹʪ��ĺ�ɫʯ����ֽ����ɫ������Ϊ���������ӷ���ʽΪCN-+H2O2+H2O=HCO3-+NH3����
(5)�����У����Һʹ��Na[Ag(CN)2]���������Ϸ����õ��ӵĻ�ԭ��Ӧ������[Ag(CN)2]-+e-=Ag+2CN-����ҵ�����ʹ�������ӣ�����Ag+��CN-���Խ�ϳ��ȶ����������Կ���������Ũ�ȣ�ʹ�Ʋ�������


 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д� Сѧ�������Ծ�ϵ�д�
Сѧ�������Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ۺ�������[Fex(OH)y(SO4)z]����Ԫ�ػ��ϼ�Ϊ+3����һ�ָ�Ч�Ļ������������ھ�ˮ��������ɿ���ͨ������ʵ��ⶨ���ٳ�ȡһ�������ľۺ����������100.00 mL����Һ����ȷ��ȡ25.00 mL��Һ�����������ữ��BaCl2��Һ��������ȫ�����ˡ�ϴ�ӡ����������أ��õ���ɫ����6.99g����ȷ��ȡ25.00mL��Һ������������NaOH��Һ��������ȫ�����ˡ�ϴ�ӡ�������������أ��õ�����ɫ����1.92g���þۺ������������x��y��z��ֵΪ
A. 6��8��5 B. 4��2��5 C. 1��1��2 D. 6��3��5
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������Ǵ�����Ҫ��Ⱦ��ɲ���ǿ�����������ѳ����ȷֽ�ȷ��������������
����֪:

(1)д����Ӧ1�����ӷ���ʽ___________��
(2)�ڷ�Ӧ2��,NO2���ij�ʼŨ��Ϊ0.1mol��L��1,��ӦΪNO2��+S2O82��+2OH-![]() NO3��+2SO42��+H2O����ͬ�¶��£��ﵽƽ��ʱNO2�����ѳ������������(Na2S2O8)��ʼŨ���Ĺ�ϵ����ͼ��ʾ��
NO3��+2SO42��+H2O����ͬ�¶��£��ﵽƽ��ʱNO2�����ѳ������������(Na2S2O8)��ʼŨ���Ĺ�ϵ����ͼ��ʾ��

�ٱȽ�a��b��ķ�Ӧ����:va��_______vb��(���>����<����=��)
�������¶ȵ����ߣ��÷�Ӧ�Ļ�ѧƽ�ⳣ��K______(����������䡱��С��)��
����֪90��ʱ��Kw=3.6��10��13����b���Ӧ��pHΪ12,����¶���K=_____(����һλС��)��
(3)��ҵ��������ƺ�����Ļ��Һ�Ʊ���������(Na2S2O8),�����ĵ缫��ӦʽΪ_______��
��N2O�ڽ�۱��淢���ȷֽ⣺2N2O(g)=2N2(g)+O2(g) ��H��
�ش���������:
(4)��֪:2NH3(g)+3N2O(g)=4N2(g)+3H2O(1) ��H1
4NH3(g)+3O2(g)=2N2(g)+6H2O(1) ��H2
��H=_____��(����H1����H2�Ĵ���ʽ)
(5)ij�¶���,���c(N2O)��ʱ��t�仯��ϵ��ͼ��ʾ��
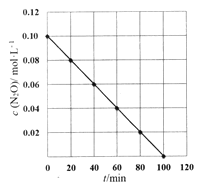
��֪˲ʱ��Ӧ����v��c(N2O)�Ĺ�ϵΪv=kcn(N2O)(k�Ƿ�Ӧ���ʳ���),��k=________,n=_____.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֬������Ӫ�����ʺ���Ҫʳ�Ҳ��һ����Ҫ�Ĺ�ҵԭ�ϡ�����������֬Ϊ��Ҫԭ�ϻ�ò��ֲ�Ʒ�ĺϳ�·��:

��֪����G (C10H10O4)�����еĹ����Ŵ��ڶ�λ;
��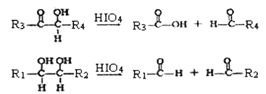
(R1��R2��R3��R4�������⡢��������������)
�ش���������:
(1)���й�����֬��˵����ȷ����____��(����)
a.��֬����ֲ���ͺ�֬������������
b.��Ȼ��֬�ǻ�ϸ�������ɵĻ����̶����۵�ͷе�
c.��֬����Ȼ�߷��ӻ����������֬����ϩ���Ļ�ѧ����
d.Ӳ�����ֽ�����֬�������ڴ�������䣬�����ױ�������������
(2)G�й����ŵ�����Ϊ______,��Ӧ�ٵķ�Ӧ����Ϊ_________��
(3)��ϵͳ������д��F������___________��
(4)����������ʵ�����B�뱽�ӷ�Ӧ����һ�����ͽṹ�߷��ӵĻ�ѧ����ʽΪ______________________��
(5)��Ԫȡ�����㻯����H��G��ͬ���칹�壬H��������������
���ܷ���������Ӧ
������������ˮ��������ʵ���֮��Ϊ2:1
�۲���NaHCO3��Һ��Ӧ��
���������������H����______��(����������ṹ��������G����)�����к˴Ź�������Ϊ�����Ľṹ��ʽΪ________(д��һ�ּ���)��
(6)д����HOCH2CH2OH![]() HCOOCH2CH2OOCH�ĺϳ�·��(���Լ���ѡ���ϳ�·�߲������е���д��ʽ)______________��
HCOOCH2CH2OOCH�ĺϳ�·��(���Լ���ѡ���ϳ�·�߲������е���д��ʽ)______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ijɷֿɼ���Ca2Mg5Si8O22(OH)2���������������ʽ�ɱ�ʾΪ�� ��
A.CaO��MgO��SiO2��H2O
B.2CaO��5MgO��8SiO2��H2O
C.2CaO��MgO��SiO2��H2O
D.5CaO��2MgO��8SiO2��H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ����٤��������ֵ������˵����ȷ����
A. 22.4L CO2������Na2O2��ȫ��Ӧ��ת�Ƶ�����Ϊ2NA
B. 6.4 g ��S2��S4��S8��ɵĻ���ﺬ��ԭ����Ϊ0.2NA
C. 1mol/L��NaCl��Һ��Cl������ĿΪNA
D. ����£�22.4L NO��11.2L O2����Ϻ����������ΪNA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ��װ�ý�����Ӧʵ�飬װ����ȷ���ܴﵽʵ��Ŀ�ĵ���

A. ��ͼa��ʾװ����֤H2��Cl2��ȼ�յ�ʵ������
B. ��ͼb��ʾװ�ôӱ���ʳ��ˮ����ȡ�Ȼ���
C. ��ͼc��ʾװ��̽�������ԣ�KMnO4��Cl2��I2
D. ��ͼd��ʾװ�÷ֽ�MgCl2��6H2O��ȡMgCl2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ijNaOH��Һ��ͨ��CO2�����õ���ҺM����CO2ͨ�������ͬ����ҺM�����Ҳ��ͬ��������ҺM����μ������ᣬ�������������V(CO2)�������������V(HCl)�Ĺ�ϵ��ͼ��ʾ�������з������жϲ���ȷ����(����CO2�ܽ�)
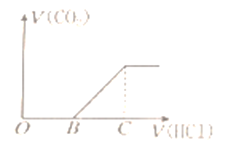
A. ��OB=0�����γ���Һ�Ĺ�������������Ӧ�����ӷ���ʽΪOH�D+CO2�THCO3�D
B. ��OB=BC������ҺMΪNa2CO3��Һ
C. ��OB��BC������ҺM�д������ڵ�������ΪCO32�D��HCO3�D
D. ��3OB=BC������ҺM��c(NaHCO3)=2c(Na2CO3)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Һ�д��ڵ���ƽ��CH3COOH![]() H����CH3COO�������������������
H����CH3COO�������������������
A. ����Һ�м�������CH3COONa���壬ƽ�������ƶ���c(CH3COO��)����
B. 0.10mol/L��CH3COOH��Һ�м�����ˮ��ƽ�������ƶ�����������ǿ
C. ͨ������NH3���壬c(H+)��С��c(OH��)����
D. 25��ʱ����������Ũ���ᣬƽ�������ƶ�������ĵ���̶ȼ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com