
����Ŀ��(1)��ϵͳ��������д�����л�������ƣ�![]() ������_____________________��
������_____________________��
(2)д��1,3-����ϩ��������ˮ��Ӧ�ķ���ʽ��_______________________________��
(3)������CH3CH3 ��CH2=CH2 ��CH3CH2C![]() CH ��CH3C
CH ��CH3C![]() CCH3 ��C2H6 ��CH3CH=CH2�У�һ����Ϊͬϵ�����________��һ����Ϊͬ���칹�����(����)________��
CCH3 ��C2H6 ��CH3CH=CH2�У�һ����Ϊͬϵ�����________��һ����Ϊͬ���칹�����(����)________��
(4)ij�л���2.3����ȫȼ�պ�����4.4�˶�����̼��2.7��ˮ����ø��л���������ܶ���2.054g/L(��״��)�����л���ķ���ʽΪ______����д������ܵĽṹ��ʽ������____________________��
���𰸡�3,4-�������� CH2=CH-CH=CH2+2Br2��CH2Br-CHBr-CHBr-CH2Br �ڢ� �ۢ� C2H6O CH3CH2OH�Ҵ���CH3OCH3����
��������
(1)��������ϵͳ������ԭ��������
(2) 1,3-����ϩ��������ˮ�����ӳɷ�Ӧ������1,2,3,4-���嶡�飻
(3)ͬϵ���ǽṹ���ơ��������������ɸ�CH2ԭ���ŵ��л��ͬ���칹���Ƿ���ʽ��ͬ���ṹ��ͬ���л��
(4)���������غ�����л�����C��H��Oԭ�����ȣ��ٸ�����Է�������ȷ�������ʽ�����ݼۼ�������д���ܵĽṹ��ʽ��
(1)��������ϵͳ������ԭ��![]() ������3,4-�������飻
������3,4-�������飻
(2) 1,3-����ϩ��������ˮ�����ӳɷ�Ӧ������1,2,3,4-���嶡�飬��Ӧ����ʽ��CH2=CH-CH=CH2+2Br2��CH2Br-CHBr-CHBr-CH2Br��
(3) C2H6�Ľṹ��ʽ��CH3CH3���٢���ͬһ���ʣ� CH2=CH2��CH3CH=CH2������̼̼˫��������������һ��CH2�����Ԣڢ�����ͬϵ�CH3CH2C![]() CH��CH3C
CH��CH3C![]() CCH3����ʽ��ͬ��̼̼������λ�ò�ͬ�����Ԣܻۢ�Ϊͬ���칹�塣
CCH3����ʽ��ͬ��̼̼������λ�ò�ͬ�����Ԣܻۢ�Ϊͬ���칹�塣
(4) 2.3���л�����ȫȼ�պ����ɵ�CO2�����ʵ�����0.1mol������ˮ�����ʵ�����0.15mol������л�������ԭ�ӵ����ʵ�����![]() ����˸��л�������ʽ��C2H6O�����л���������ܶ���2.054g/L�����Ը��л����Ħ��������2.054g/L��22.4L/mol=46g/mol��������Է���������֪����ʽҲ��C2H6O�����ܵĽṹ��ʽ��CH3CH2OH�Ҵ���CH3OCH3���ѡ�
����˸��л�������ʽ��C2H6O�����л���������ܶ���2.054g/L�����Ը��л����Ħ��������2.054g/L��22.4L/mol=46g/mol��������Է���������֪����ʽҲ��C2H6O�����ܵĽṹ��ʽ��CH3CH2OH�Ҵ���CH3OCH3���ѡ�


 ����˼ά�żӿ���ϵ�д�
����˼ά�żӿ���ϵ�д� �����Ծ�ϵ�д�
�����Ծ�ϵ�д� �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮ����п����𩷯��������ýȾ������������ľ�ķ���������ҵ��������п��(��Ҫ�ɷ�ΪZnO������ZnSiO3��FeCO3��CuO��)����ZnSO4��7H2O���������£�
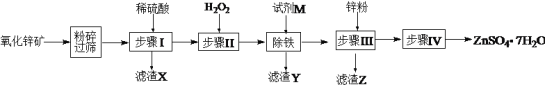
�ڸ������У���������������������pH�����
��ʼ����ʱpH | ��ȫ����ʱpH | |
Zn2+ | 5.4 | 6.4 |
Fe3+ | 1.1 | 3.2 |
Fe2+ | 5.8 | 8.8 |
Cu2+ | 5.6 | 6.4 |
��ش��������⣺
(1)��������п��ʯ��Ŀ����______________������X�ijɷ���________________��
(2)������м���H2O2Ŀ���ǣ�_______________��������Ӧ�����ӷ���ʽΪ��______________��
(3)�������������м����Լ�M������Һ��pH���Լ�M������________(�ѧʽ��һ�ּ���)��������Һ��pH��ΧΪ��_________��ͬʱ����Ҫ����Һ���ȣ���Ŀ���ǣ�__________��
(4)����Z�ijɷ���____________��
(5)ȡ28.70 g ZnSO4��7H2O(��Է���������287)��������ͬ�¶ȣ�ʣ�����������仯��ͼ��ʾ��
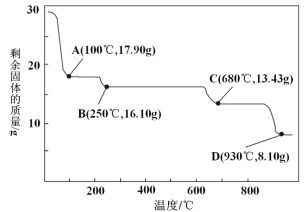
�ٲ�����еĺ�ɲ������ڼ�ѹ�����½��У���ԭ����____________��
��680 ��ʱ���ù���Ļ�ѧʽΪ________________��
a.ZnO b.Zn3O(SO4)2 c.ZnSO4 d.ZnSO4��H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������и�ʵ��װ�õ������У�����ȷ���ǣ� ��
A. ������ʵ������ȡ����NH3��O2
������ʵ������ȡ����NH3��O2
B. ���ô�a����ˮ�ķ�������װ������������
���ô�a����ˮ�ķ�������װ������������
C. ʵ���ҿ���װ�����ռ�H2��NH3
ʵ���ҿ���װ�����ռ�H2��NH3
D.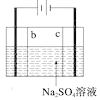 ��������������ƣ�����bΪ�����ӽ���Ĥ��cΪ�����ӽ���Ĥ
��������������ƣ�����bΪ�����ӽ���Ĥ��cΪ�����ӽ���Ĥ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijС��ͬѧ����ͼ1װ��(�г�������ʡ��)�Ʊ��屽����̽���÷�Ӧԭ����
��.�Ʊ��屽
(1)װ���г�����a��������__________���������塣
(2)��ʵ������õ��屽Ϊ��ɫ������Ϊ_________.
��.�����ᴿ
��֪���屽�뱽���ܣ�Һ�塢�����屽�ķе�����Ϊ59�桢80�桢156�档ͬѧ���������ͼ2���̣�
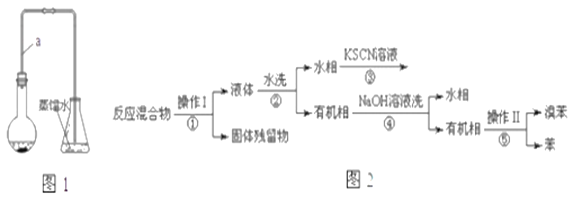
(3)������Ϊ________.
(4)���̢ں͢��У���Ҫ�õ��IJ����������ձ���___�����̢۵�����Ϊ____�����̢ܵ�������_____.
��.̽��ԭ��
(5)��Ӧ��������ijͬѧȡ������ƿ�е�Һ�����Թ������ٵ��뼸��AgNO3��Һ������dz��ɫ�������Ӷ��жϷ�Ӧһ��������HBr������Ϊ��ͬѧ���ж�________(����������������������)��
(6)Ϊ̽����ƿ����Һ����������������̽��ʵ�鷽��(��ѡ�Լ���þ�ۡ����Ȼ�̼����ˮ����ˮ������ˮ)
ʵ�鲽�� | Ԥ������ | ���� |
1.����ƿ�е�Һ��ת���Һ©������������__�����Һ���ֱ�ȡ������_����Һ���Թ�A��B�� | _______ | ______ |
2.���Թ�A�м���������___�����Ȼ�̼�������� | ��Һ�ֲ����²��Ԣ�___ɫ | ��ƿ��Һ�庬����Br |
3.���Թ�B�м����___. | ���������� | ��ƿ��Һ�庬������___ |
�������������ƶϣ��Ʊ��屽�ķ�Ӧ����___��Ӧ���䷴Ӧ����ʽΪ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ�кܶ���Ҫ��ԭ�϶�����Դ��ʯ�ͻ������ش���������

��1��A���ṹ��ʽΪ__________________
��2����ϩ�������������ŵ�����Ϊ__________________
��3��д����Ӧ�ٵķ�Ӧ���ͣ�_____________
��4��д��������ͼ��ijЩ���̵Ļ�ѧ����ʽ��
���̢�_________________________
���̢�______________________
���̢�_________________
��5�������й�ʵ���˵������ȷ����_______
A. ��ȥ���������е����ᣬ�ɼ���NaOH��Һ�����÷�Һ
B. �л���C���ϩ������ͬϵ��
C. �۱�ϩ���ܹ�ʹ���Ը��������Һ��ɫ
D. ��ȥ�������л��е�����ŨHNO3��H2SO4���ɽ��䵹�뵽һ������NaOH��Һ�У����÷�Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ����С����ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A(A�¶˻����ر�)�С�

(1)д��A�з�Ӧ�Ļ�ѧ����ʽ_______________��
(2)�۲쵽A�е�������_____________________��
(3)ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������Ŀ����________��д���йصĻ�ѧ����ʽ______________________________��
(4)C��ʢ��CCl4��������________________��
(5)��֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�м���AgNO3��Һ������������ɫ����������֤������һ����֤�ķ��������Թ�D�м���_______��������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ����ͬ�����£������ݻ���ͬ���Թֱܷ�װ��NO2��NO���壬�ֱ�����ˮ���У�Ȼ��ͨ�����ܻ���ͨ����������ͨ������ҡ���Թܣ�ֱ�������Թ��ڳ���Һ�塣�����Թ��ڵ����ʲ���ˮ������ɢ���������Թ�����Һ���ʵ���Ũ��֮��Ϊ
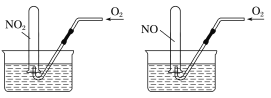
A.1��1B.5��7C.7��5D.4��3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҩ��Ƴɷ��к���������ͪ�����ʡ��ӷ��͡��л��ӡ����ǵ�����ɷ֣�����һ���л��ӵĽṹ��ʽ��ͼ������˵����ȷ���� ( )
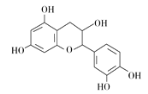
A. ����������̼ԭ�ӿ��Դ���ͬһƽ��
B. ����ʽΪC15H12O7
C. 1 mol���л��������Ũ��ˮ��Ӧ���������5 mol Br2
D. 1 mol���л�����NaOH��Һ��Ӧ�������5 mol NaOH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪H2C2O4ˮ��Һ��H2C2O4��HC2O4-��C2O42-������̬�����ӵ����ʵ����������ֲ�ϵ����������ҺpH�仯�Ĺ�ϵ��ͼ��ʾ������˵����ȷ����
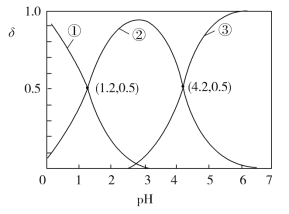
A.������������������HC2O4-
B.H2C2O4��Ka1=-1.2
C.�������Һ�еμ�KOH��Һ��pH=4.2��c(K+)��3c(C2O42-)
D.Ũ�Ⱦ�Ϊ0.01 mol��L1�IJ�����KOH��Һ�������ϲ���ַ�Ӧ�õ�����Һ��c(K+)��c(HC2O4-)��c(H2C2O4)��c(C2O42-)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com