
����Ŀ����ͼ��ʾ��ijͬѧ���һ��ȼ�ϵ�ز�̽���ȼҵԭ���ʹ�ͭ�ľ���ԭ����������װ����XΪ�����ӽ���Ĥ��

����Ҫ��ش�������⣺
��1��ͨ�������ĵ缫Ϊ___�������������������������������ĵ缫��ӦʽΪ__��
��2��ʯī�缫(C)�缫Ϊ___��������������������������Fe�缫�ĵ缫��ӦʽΪ__��
��3����Ӧһ��ʱ�����װ������������������Ҫ��__��������������������ʯī������
��4�������ͭ�к���п���������ʣ����װ���з�Ӧһ��ʱ�������ͭ��ҺŨ�Ƚ�__����������������С����������������
��5�����ڱ�״���£���4.48L�����μӷ�Ӧ������װ�������缫�����ɵ�����ķ�����Ϊ__����װ������������ͭ������Ϊ__��
���𰸡����� O2+4e-��2H2O=4OH- ���� 2H++2e-=H2�� ���� ��С 2.408��1023��0.4NA 25.6g
��������
ԭ��صĸ�������������Ӧ������������ԭ��Ӧ����˼׳��е�ͨ�������ĵ缫Ϊ������ͨ�������ĵ缫Ϊ���������������Դ����������Ϊ���������Դ����������Ϊ��������ˣ��ҳ���Fe�缫Ϊ������C�缫Ϊ�����������У���ͭΪ��������ͭΪ������
(1)ͨ��������֪��ͨ�������ĵ缫Ϊ����������ͨ����������������ĵ缫��ӦʽΪ��![]() ��
��
(2)ͨ��������֪���ҳ���C�缫Ϊ������Fe�缫Ϊ�����������缫��ӦʽΪ��![]() ��H+����ˮ��������ģ�
��H+����ˮ��������ģ�
(3)ͨ��������֪���ҳ���Fe�缫Ϊ����������������![]() ��������ˮ�������ɵ�H+�������������������NaOH��
��������ˮ�������ɵ�H+�������������������NaOH��
(4)ͨ��������֪�������д�ͭΪ���������ڴ�ͭ�к������ʣ����Ե��ʱ������һ����Cu������ΪCu2+��������ʼ����Cu2+����ԭΪCu���ʣ����Ե������Һ��Cu2+Ũ�Ȼ��½���
(5)�����4.48LO2��0.2mol���ܹ�ת��0.8mol���ӣ��ҳ���Fe�缫����H2�����������0.4molH2��0.4NA�����ӣ���װ�õľ�ͭ�缫��������0.4molͭ���ʣ���25.6gͭ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����仯�����ڹ�ũҵ�����о�����Ҫ��;���ش��������⣺
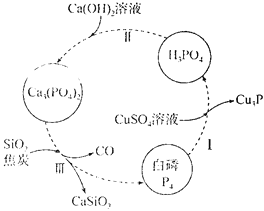
(1)��ͼ��ʾΪ�ᴿ������Ʒ(����������)�Ĺ������̡�����I�У�����ԭ��Ԫ����________(��Ԫ�ط���)������III�Ļ�ѧ����ʽΪ__________��
(2)���ᷰ�/̼���ϲ���[Li3V2(PO4)3/C]�dz��õĵ缫���ϣ����Ʊ��������£�
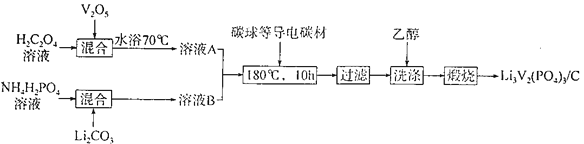
�ٸ��ϲ�����V�Ļ��ϼ�Ϊ________��C��������____________��
��V2O5��H2C2O4��Ӧ����V2(C2O4)3�Ļ�ѧ����ʽΪ____________����ϴ����ʱ���Ҵ�������ˮ��Ŀ����________________��
������ӵ����һ�ֶ��ε�أ��ֳ���ҡ������ء����ú�LixC6��Li3V2(PO4)3/C���缫���ŵ�ʱ�ĵ���ܷ�ӦΪLixC6��Li3��xV2(PO4)3= Li3V2(PO4)3+C6�����س��ʱ�����ĵ缫��ӦʽΪ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ŀ��һ���¶��£���ӦN2(g)+3H2(g)![]() 2NH3(g)�ﵽ��ѧƽ��״̬�ı�־��
2NH3(g)�ﵽ��ѧƽ��״̬�ı�־��
A. c(N2):c(H2):c(NH3)=1:3:2
B. N2��H2��NH3�����ʵ����������ٸı�
C. N2��H2�����ʵ���֮����NH3�����ʵ�����2��
D. ��λʱ����ÿ����lmolN2��ͬʱ����3molH2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪X��Y��Z��W����Ԫ����Ԫ�����ڱ�������������ͬ�����ڵ�Ԫ�أ���ԭ��������������X��Wͬ���壬Y��ZΪͬ���ڵ�����Ԫ�ء�Wԭ�ӵ�����������Y��Zԭ������������֮�͡�Y���⻯���������3�����ۼ���Zԭ�������������Ǵ�����������3�������ƶϣ�
��1��X��Y��Z��W����Ԫ�صķ��ţ� X________��Y__________��Z____________��W��________
��2��������Ԫ���е�����Ԫ����ɵ�������ˮ��ˮ��Һ�Լ��ԵĻ�����ĵ���ʽ�ֱ�Ϊ__________________��______________________��
��3����X��Y��Z���γɵ����ӻ�������________________������W������������ˮ�����Ũ��Һ����ʱ��Ӧ�����ӷ���ʽ��________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���о���ѧ��Ӧ�е������仯����Ҫ���塣�����ѧ����֪ʶ�ش��������⣺
��.��ѧ��Ӧ����Ϊ�ɼ����Ѻ��¼��γɵĹ��̡���ѧ���ļ������γ�(���)1 mol��ѧ��ʱ�ͷ�(������)����������֪��N��N���ļ�����948.9 kJ��mol��1��H��H���ļ�����436.0 kJ��mol��1��N��H���ļ�����391.55 kJ��mol��1����N2(g)��3H2(g)=2NH3(g)����H��______________
��.��ͼ��þ��ԭ�����д���ĵ���ܷ�Ӧ______________ ���ҳ������ĵ缫��Ӧʽ______________

��.��ͼ��һ����ѧ���̵�ʾ��ͼ���ش��������⣺

��1����װ���е缫A��������__________
��2����װ����ͨ��CH4�ĵ缫��ӦʽΪ ________________________________________________
��3��һ��ʱ�䣬�������в���224 mL(��״����)����ʱ�����Ƚ�����أ�������Һ��25 ��ʱ��pH��________(��֪��NaCl��Һ������������Һ���Ϊ1000 mL)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ԫ��W��X��Y��Z��ԭ���������ε�����a��b��c��d��e��f������ЩԪ�سɵĻ�������d�ǵ���ɫ��ĩ��mΪԪ��Y�ĵ�����ͨ��Ϊ��ɫ��ζ�����塣�������ʵ�ת����ϵ��ͼ��ʾ������˵���������

A. �����Ӱ뾶��Z<Y
B. d�мȺ������Ӽ��ֺ��й��ۼ��� ���������Ӻ������ӵ���Ŀ֮��Ϊ2:1
C. ����̬�⻯������ȶ��ԣ�Y>X
D. ������4��Ԫ����ɵĻ������ˮ��Һһ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ӵ�����ˮ����ȡ����������£�

�����й�˵������ȷ����(����)
A.X����![]() ������Һ
������Һ
B.������������ӷ�ӦΪ![]()
C.��ҵ��ÿ���1 mol![]() ��������Ҫ���ı�״��
��������Ҫ���ı�״��![]() 22.4 L
22.4 L
D.������漰�IJ�������ȡ����Һ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�������ﺬNH4I��NaHCO3��AlCl3��MgBr2��FeCl2�еļ���,Ϊȷ���ù�������ijɷּ�����ɳɷֵ����ʵ���֮��,�ֽ�������ʵ�顣
ʵ����:

(1)��ɫ����Ϊ_______��
(2)�ù�������ijɷ�Ϊ_______��
ʵ����:ȡһ�����ĸù�����������ˮ���1 L��Һ,����û����Һ��ͨ��һ������Cl2,�����Һ�м���������(�ֱ���A-��B-��C-��ʾ)�����ʵ�����ͨ��Cl2����Ĺ�ϵ�����ʾ��

(3)a=____��
(4)ԭ���������и���ɳɷֵ����ʵ���֮��Ϊ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ö��Ե缫��⣬�����ӽ���Ĥ����ⱥ��ʳ��ˮ�����ۺ��ܺĵ͡�������ȾС���ŵ㡣������������ͼ��ʾ��

(1)��ⱥ��ʳ��ˮ�Ļ�ѧ����ʽΪ______��
(2)���������ܹ���ȥ����Һ�������ȵ��Լ�����_________(����ĸ���)��
a��Na2SO4b��Na2SO3 c���ȿ�������d������������Һ���Ϸ�����ѹ
(3)ʳ��ˮ�е�I-��������ۣ��ɱ���������Cl2����ΪICl������һ��ת��ΪIO3-��IO3-�ɼ���������Ϊ�ߵ����(IO4-)����Na+��������ܽ�Ƚ�С��NaIO4�����������ӽ���Ĥ�ϣ�Ӱ��Ĥ��������
�ٴ�ԭ�ӽṹ�ĽǶ��ж�ICl�е�Ԫ�صĻ��ϼ�ӦΪ________��
��NaIO3������ΪNaIO4�����ӷ���ʽΪ_________��
(4)�����������¼���NaClO��Һ���ɽ�ʳ��ˮ�е�I-ת��ΪI2���ٽ�һ����ȥ��ͨ���ⶨ��ϵ������ȣ����Լ�ⲻͬpH��I2����������ʱ��ı仯����ͼ��ʾ����֪�������Խ�߱�������ϵ��c(I2)Խ��

�����ӷ���ʽ����10minʱ��ͬpH��ϵ����Ȳ�ͬ��ԭ��______��
��pH=4.0ʱ����ϵ������Ⱥܿ�ﵽ���ֵ��֮������½�������ȿ����½��Ŀ���ԭ��______��
���о�����ʳ��ˮ��I-������0.2mgL-1ʱ�����ӽ���ĤӰ��ɺ��ԡ��ֽ�1m3��I-Ũ��Ϊ1.47mgL-1��ʳ��ˮ���д�����Ϊ�ﵽʹ�ñ���������������Ҫ0.05molL-1NaClO��Һ___L��(��֪NaClO�ķ�Ӧ����ΪNaCl����Һ����仯���Բ���)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com