
��֪�ں�HNO3����Һ�з���Al������H2��ij��ɫ��Һ�ֻ��������11�������еļ��֣�Mg2����Fe3+��H����Ag����Ba2����SO42����HCO3����OH����MnO4����NO3����CO32������֪����Һ�ܸ���������Ӧ���ҷų�������ֻ���������Իش�
��1������Һ������Ӧֻ��AlO2�����ɣ���ԭ��Һһ�����еĴ�����������________(�ѧʽ)��������Ӧ�����ӷ���ʽ��_____________________________________________________�������ܺ��еĽ϶��������__________________(�ѧʽ)��
��2������Һ������Ӧ����Al3�����ɣ���ԭ��Һ�п��ܣ�����һ���ܣ��������ڵ�������_____________��
��1��Ba(OH)2 2Al��2OH����2H2O=2AlO2����3H2�� Ba(NO3)2 ��2��H+ SO42- Mg2+
�����������������Һ�ܸ���������Ӧ���ҷų�������ֻ����������˵������Һ���������ԣ�Ҳ�����Լ��ԣ�����Ϊ����Һ����ɫ�ģ�����һ��û��Fe3+��MnO4�����ݴ˿����жϡ�
��1������Һ������Ӧֻ��AlO2�����ɣ���˵����һ���Լ��ԣ���Mg2����H����Ag����HCO3��һ�����ܴ������ڡ�������Һ�ĵ����Կ�֪��һ������Ba2�������Ծ�һ��û��SO42����CO32�������ԭ��Һһ�����еĴ�����������Ba(OH)2����NO3������ȷ�������Ի����ܺ��еĽ϶��������Ba(NO3)2���йط�Ӧ�����ӷ���ʽΪ2Al��2OH����2H2O=2AlO2����3H2����
��2������Һ������Ӧ����Al3�����ɣ���˵������Һһ�������ԡ���HCO3����OH����CO32��һ�����ܴ������档����Ϊ�ں�HNO3����Һ�з���Al������H2�����Ը���Һ��Ҳһ�����ܴ�������NO3����������Һ�ĵ����Կ�֪����Һ��һ������SO42�������Ծ�һ�����ܴ�������Ag����Ba2��������ԭ��Һ�п��ܣ�����һ���ܣ��������ڵ�������)H+��SO42-��Mg2+��
���㣺�������ӹ����Լ����Ӽ�����й��ж�


 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������������A��B��C��D��E�����ǵ������ӿ�����Na+��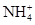 ��Cu2+��Ba2+��Al3+��Ag+��Fe3+�������ӿ�����Cl-��
��Cu2+��Ba2+��Al3+��Ag+��Fe3+�������ӿ�����Cl-�� ����֪��
����֪��
�������ξ�����ˮ��ˮ��Һ��Ϊ��ɫ��
��D����ɫ��Ӧ�ʻ�ɫ��
��A����Һ�����ԣ�B��C��E����Һ�����ԣ�D����Һ�ʼ��ԡ�
�������������ε���Һ�зֱ����Ba��NO3��2��Һ��ֻ��A��C����Һ������������
�������������ε���Һ�У��ֱ���백ˮ��E��C����Һ�����ɳ����������Ӱ�ˮ��C�г�����ʧ��
�ް�A����Һ�ֱ���뵽B��C��E����Һ�У��������ɲ�����ϡ����ij�����
�ش��������⣺
��1���������У�һ��û�е���������_________________________________________________��
��������������ͬ�������εĻ�ѧʽ��_____________________________________________��
��2��D�Ļ�ѧʽΪ_________��D��Һ�Լ��Ե�ԭ���ǣ������ӷ���ʽ��ʾ��________________��
��3��A��C����Һ��Ӧ�����ӷ���ʽ��______________________________________________��
E�Ͱ�ˮ��Ӧ�����ӷ���ʽ��______________________________________________________��
��4����Ҫ����B�������������ӣ���ȷ��ʵ�鷽����__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�弰�仯����㷺Ӧ�����л��ϳɡ���ѧ����������
��1����ˮ�����������Ԫ�صı仯���£�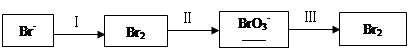
�ٹ��̢�ˮ�Լ��ԣ�����pH��3.5����ͨ��������
��.ͨ��������Ӧ�����ӷ���ʽ��______��
��.����ˮpH�����Cl2�������ʣ���ƽ��ԭ��������ԭ����______��
�ڹ��̢����ȿ�������ϳ�������Ũ̼������Һ���ա���ɲ���ƽ���з���ʽ��
Br2�� Na2CO3��
Na2CO3�� NaBrO3��
NaBrO3�� CO2��
CO2�� ______
______
�۹��̢��������ữ�ɵ�Br2��Na2SO4�Ļ����Һ��
��ͬ�����£����������ữ������������������٣�ԭ����______��
��2��NaBrO3��һ�ַ����Լ����������ữ��NaI��Һ����μ���NaBrO3��Һ��������2.6 mol NaBrO3ʱ����÷�Ӧ����Һ����͵�Ĵ�����ʽ�����ʵ����ֱ�Ϊ��
| ���� | I2 | Br2 | IO3- |
| ���ʵ���/mol | 0.5 | 1.3 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������ʣ��� �������ƹ��壻�� ͭ˿���� �Ȼ������壻�� ϡ����� ������̼���壻 ������������Һ���� ̼���Ʒ�ĩ���� ���Ǿ��壻�� �����Ȼ��ƣ����������գ�
��1������״̬�¿ɵ������________����2�����ڵ���ʵ���________����3�����ڷǵ���ʵ���________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����������ʣ���NaCl���壻��Һ̬SO2���۴���������ᱵ������ͭ���ƾ���C2H5OH�������ۻ���KCl����NaOH��Һ��
�����������ʻش��������⡣������ţ�
��1��������״̬���ܵ��������������������������
��2������������ʵ�������������������
��3�����ڷǵ���ʣ�������ˮ���ˮ��Һ�ܵ��������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ű�Ҫ��д�����з�Ӧ�����ӷ���ʽ
��������ͭ��������
��̼������Һ�к�������������Һ���
��2�������������ʣ���NaCl���� ��Һ̬HCl ��CaCO3���� ������KCl �����Ǣ�ͭ��CO2��H2SO4��KOH���� ��ˮ�������CH3COOH �Ѿƾ�������ţ�
�������������ܵ������_________________________________��
���������������ڵ���ʵ���_____________________________��
���������������ڷǵ���ʵ���___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������������:
���� ������ ��CO2 ��H2SO4 ��Ba(OH)2 ���ɫ�������������� ��HCl
��1���������������ڵ���ʵ��� ��(�����)
��2�������Һ���μӢߵ���Һ,������������ ��
��3������������������������ˮ��Һ�з�����Ӧ,�����ӷ���ʽΪ:H++OH-=H2O,��÷�Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ҫ������������
(l)д���������ʵĵ��뷽��ʽ��
Fe2(SO4)3__________________________________________________��
NaHCO3______________________________________________________��
(2)д�����з�Ӧ�����ӷ���ʽ��
ϡ������̼��Ʒ�Ӧ____________________________________________��
����������Һ��ϡ���ᷴӦ_______________________________________��
(3)д�����������ӷ���ʽ���Ӧ�Ļ�ѧ����ʽ��
H����OH��=H2O __________________________________________��
CO32����2H��=CO2����H2O__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijNa2CO3��NaAlO2�Ļ����Һ����μ���1 mol��L-1������,�����Һ�е�C ��HC
��HC ��Al
��Al ��Al3+�����ʵ�����������������仯��ϵ��ͼ��ʾ,������˵����ȷ���ǣ� ��
��Al3+�����ʵ�����������������仯��ϵ��ͼ��ʾ,������˵����ȷ���ǣ� ��
A��ԭ�����Һ�е�C ��Al ��Al �����ʵ���֮��Ϊ1��2 �����ʵ���֮��Ϊ1��2 |
| B��V1��V2=1��5 |
| C��M��ʱ���ɵ�CO2Ϊ0.05 mol |
D��a�߱�ʾ�����ӷ���ʽΪ:Al +H++H2O +H++H2O Al(OH)3�� Al(OH)3�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com