
���� T/��n/mol | ����̿ | NO | E | F |
| ��ʼ | 2.030 | 0.100 | 0 | 0 |
| T1 | 2.000 | 0.040 | 0.030 | 0.030 |
| T2 | 2.005 | 0.050 | 0.025 | 0.025 |
|
| c(H+)��c(HCO3-) |
| c(H2CO3) |
 ��
�� ��
��
|
| Ksp |
| c(CO32-) |
| 2.8��10-9 |
| 1��10-4 |
| c(H+)��c(HCO3-) |
| c(H2CO3) |
| 10-5.6��10-5.6 |
| 1.5��10-5 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ȥCO������O2��ͨ�����ȵ�Cu�����ռ����� |
| B����ȥK2CO3����������NaHCO3�����������м��� |
| C����ȥKCl��Һ�е�����MgCl2����������NaOH��Һ������ |
| D����ȥCO2�е�����HCl��ͨ�뱥��NaHCO3��Һ���ռ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��1mol Na2O��Na2O2�Ļ������������������ΪNA |
| B��1.7g H2O2�к��еĵ�����Ϊ0.9NA |
| C��1mol Na2O2��������CO2��Ӧ������ת����Ϊ2NA |
| D����״���£�2.24L CO2��CO������������Ϊ0.1NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� ���� | IA | ��A | ��A | ��A | VA | ��A | ��A | 0 |
| �� | �� | �� | ||||||
| �� | �� | �� | �� | �� | �� | �� | ||
| �� | �� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
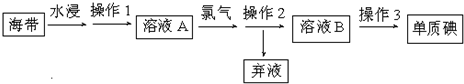
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

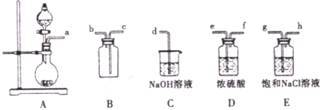
| ʵ����� | ʵ����� | ���� |
| �� | ����ˮ����Ʒ����Һ | �� |
| �� | ��ˮ�м���̼�����Ʒ�ĩ | ����ɫ���ݲ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com