
�й��ܺ�������������ʼ��±���
| ��ѧʽ | �ܶȻ�������ʱ��Ksp | ������ȫʱ��pH | �Ȼ��ܾ�������� |
| Co(OH) 2 | 5.9��10-15 | 9.4 | CoCl2��6H2O�ʺ�ɫ���������ȶ���110ºC��120ºCʱ��ˮ�����ɫ��ˮ�Ȼ��� |
| Fe(OH) 2 | 1.6��10-14 | 9.6 | |
| Fe(OH) 3 | 1.0��10-35 | x |
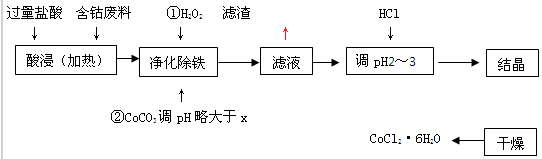
��1��CoCO3+2H+=Co2++H2O+CO2��
��2��4
��3��ʹFe3+��ȫ����
��4��CoCl2
��5����ѹ���¶ȵ���110�����HCl�����м��ȣ���д2�㣩
���������������1���������������У�����CoCO3�����ܣ���CoCO3����̼���Σ������ᷴӦ��CoCO3+2H��=Co2��+H2O+CO2����ͨ���˷�Ӧ������Һ�����ԣ�����Һ��pH����Ϊ��CoCO3+2H��=Co2��+H2O+CO2����
��2����Һ�У�Fe3������1.0��10-5mol��L��1ʱ������ΪFe3��������ȫ��Ksp[Fe(OH)3 ]=1.0��10-35=c��Fe3������c3��OH����=1.0��10-5mol��L-1��c3��OH����������c��OH����=10-10mol��L-1��c��H����=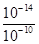 mol��L-1=10-4mol��L-1��PH=-lgc��H����=-lg10-4=4����Ϊ��4��
mol��L-1=10-4mol��L-1��PH=-lgc��H����=-lg10-4=4������4��
��3�����ݣ�2��������֪��Ϊ��ʹFe3����ȫ������pHӦ�Դ���4����Ϊ��ʹFe3����ȫ������
��4����ȥFe3������Һ����Ҫ����NaCl��CoCl2���Լ���������HCl����Ϊ��CoCl2��NaCl��
��5����CoCl2��6H2O��110��C��120��Cʱ��ˮ�����ɫ��ˮ�Ȼ��ܣ����Բ����ø��º�ɣ�Ϊ�˷�ֹCoCl2��6H2O��ˮ�������ʱ�˲��õķ���������Ǽ�ѹ���¶ȵ���110�����HCl�����м��ȣ���Ϊ����ѹ���¶ȵ���110�棻����HCl�����м��ȣ�
���㣺���ʷ�����ᴿ�ķ����ͻ��������ۺ�Ӧ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
������ʵ��װ�ý�����Ӧʵ�飬�ܴﵽʵ��Ŀ�ĵ���
| A����ͼ1��ʾװ�÷����л����ˮ�� |
| B����ͼ2��ʾװ�ô�ʳ��ˮ����ȡNaCl |
| C����ͼ3��ʾװ����ˮ������HCl |
| D����ͼ4��ʾװ���ռ������鰱�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ͼ�dz��������IJ��ֽṹ��
(1)���������A��������,B��������,C������,D����������
(2)ʹ��ǰ�����Ƿ�©ˮ������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�о�С����������в��ἰ�����εĺ���(��C2O42-��)��ʵ�鲽�����£�
�ٽ�������ƷԤ��������ˮ���ݣ����˵õ����в��ἰ�����ε���Һ��
�ڵ�����Һ������ԣ��μ�����CaCl2��Һ��������ɫ�����������������ᣬʹCaCO3�ܽ⣻���˵õ�CaC2O4���塣
����ϡHCl�ܽ�CaC2O4����ˮ����100 mL��Һ��ÿ��ȷ��ȡ25.00 mL����Һ����0.0100 mol��L��1 KMnO4����Һ�ζ���ƽ�����ı���ҺV mL��
�ش��������⣺
(1)������С���ƷԤ�������ķ����� (A.���ճɻң�B.��ĥ��֭)��
(2)������С�������Һ������ԡ��� (A.�����ԣ�B.�����ԣ�C.����)����֤CaCl2��Һ�ѡ��������IJ����������� ��
(3)��������õ��IJ����������ձ�����ƿ����ͷ�ιܡ��������⣬���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ӵ�ʳ���к��еĵ������һ�ְ�ɫ�ᾧ��ĩ�������º��ȶ���������560�濪ʼ�ֽ⡣�����������µ������һ�ֽ�ǿ��������������⻯��������εȻ�ԭ�����ʷ�Ӧ����ҵ��������ص��������£�
��1����������������ص���Ҫ������ ��
��2�������±�����ص��ܽ�ȣ������۵õ�����ؾ��壬�ɾ��� �����ˡ�ϴ�ӡ�����Ȳ��衣
| �¶�/�� | 20 | 40 | 60 | 80 |
| KIO3/100gˮ | 8.08 | 12.6 | 18.3 | 24.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����a��e����ѧ��ѧʵ���г����ļ��ֶ���������
(a)��Ͳ (b)����ƿ (c)�ζ��� (d)������ƽ (e)�¶ȼ�
��1���ޡ�0���̶ȵ��� (��д���)��
��2�����в����������� (����ĸ)
| A����25mL��ʽ�ζ�����ȡ20.00mLNaHCO3 |
| B����������ƽȷ����10.20��̼���ƹ��� |
| C����100mL��Ͳ��ȡ3.2mLŨ���� |
| D������1 mol��L�C1������������Һ475mLѡ��500mL����ƿ |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������������Ҫ��Fe2O3��SiO2��Al2O3��MgO�����ʣ��Ʊ��������Ĺ����������£�
��1���������������Ҫ�ʵ�������Ŀ���Ǣ�������Ľ����ʣ��� ��
��2������ԭ���ǽ�Fe3��ת��ΪFe2����ͬʱFeS2������ΪSO42�����÷�Ӧ�����ӷ���ʽΪ ��
��3��Ϊ�ⶨ��������������Һ��Fe3�������Կ��Ƽ���FeS2������ʵ�鲽��Ϊ��ȷ��ȡһ���������������Һ����ƿ�У�����HCl���Թ���SnCl2���ټ�HgCl2��ȥ������SnCl2���Զ�����������Ϊָʾ������K2Cr2O7����Һ�ζ����йط�Ӧ����ʽ���£�
2Fe3����Sn2����6Cl����2Fe2����SnCl62����
Sn2����4Cl����2HgCl2��SnCl62����Hg2Cl2����
6Fe2����Cr2O72����14H����6Fe3����2Cr3����7H2O��
����SnCl2����������ⶨ��Fe3���� ���ƫ�ߡ�����ƫ�͡��������䡱����ͬ����
��������HgCl2����ⶨ��Fe3���� ��
��4���ٿ�ѡ�� �����Լ���������Һ�к���Fe3+������Fe3+��ԭ���� �������ӷ�Ӧ����ʽ��ʾ����
����֪����������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Al(OH)3 | Fe(OH)2 | Mg(OH)2 | Mn(OH)2 |
| ��ʼ���� | 2.7 | 3.8 | 7.5 | 9.4 | 8.3 |
| ��ȫ���� | 3.2 | 5.2 | 9.7 | 12.4 | 9.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��������ƵĴ��ȿ��õζ������вⶨ��ԭ���ǣ�2S2O32��+ I2 �� S4O62��+ 2I-
38.����100 mL0.0500 mol/L I2��Һ������Ҫ�������� ��ѡ���ţ���
a��100 mL����ƿ b����Ͳ c���ձ� d��������
�ζ��ܱ���ʹ���¶ȣ�20oC; �ζ��ܵ���С�̶�Ϊ mL��
39.ȡ2 .500g�����ʵ�Na2S2O3��5H2O�������50mL��Һ��ÿ��ȡ10.00mL������ƿ���2�ε���Ϊָʾ��������0.0500 mol/L I2��Һ�ζ���ʵ����������(��3�γ�����Ϊ 0.00���յ������ͼ; ���ʲ��μӷ�Ӧ)��
| ��� | 1 | 2 | 3 |
| ����I2��Һ�����/mL | 19.98 | 20.02 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�Ȼ��Ƴ����ڵ�·�ڱ��������������������ˮ�������������ʡ�ʵ�����ù�ҵ����ʯ����������Al2O3��Fe2O3�����ʣ��Ʊ��Ȼ��Ƶ���Ҫ�������£� ���������գ�
���������գ�
��1������ʹ�õ���������ʵ���Ũ��ԼΪ6.0mol/L������36.5%�����ᣨ�ܶ�Ϊ1.2g/mL������6.0mol/L������100mL������IJ��������в���������Ͳ����ͷ�ιܡ� ����Ҫ��ȡ36.5%������ mL�����ƹ����У���������������ȷ�����в���������Ũ��ƫС���� ��
| A������ҡ�Ⱥ���Һ����ڿ̶��� |
| B������ʱ��������ƿ�Ŀ̶��� |
| C������Һת������ƿ��û��ϴ���ձ��Ͳ���������ת�붨�ݲ��� |
| D�������ˮ�����˿̶��ߣ�ȡ������ˮʹҺ��ǡ�õ��̶��� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com