
��ͼ�У�A��B��C��D��E�ǵ��ʣ�G��H��I��F��B��C��D��E�ֱ��A�γɵĶ�Ԫ�������֪��
�ٷ�ӦC+G B+H�ܷų��������ȣ��÷�Ӧ��Ӧ��������ĺ��ӣ�
B+H�ܷų��������ȣ��÷�Ӧ��Ӧ��������ĺ��ӣ�
��I��һ�ֳ������������壬����E���Է�����Ӧ��2E+I 2F+D��F��EԪ�ص���������Ϊ60%��
2F+D��F��EԪ�ص���������Ϊ60%��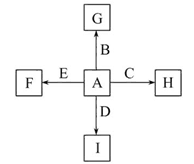
�ش����⣺
��1�����з�Ӧ�Ļ�ѧ����ʽΪ��________________________________________��
��2��1.6 g G�������ᣬ�õ�����Һ��ͭ����ȫ��Ӧ������������ͭ�۵�������д�����ӷ���ʽ�ͼ�����̣���__________________________________________��
��3��C�����NaOH��Һ��Ӧ�����ӷ���ʽΪ��___________________________����Ӧ����Һ�����������I��Ӧ�����ӷ���ʽΪ��_________________________��
��4��E��I��ȼ�չ۲쵽�������ǣ�____________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ͻ��ǽ��캽��ĸ����������ϡ�
(1)��ĸ�������������Ͻ����졣
����Ԫ�������ڱ��е�λ��Ϊ________����ҵ������ԭ������������ȡ���ã���ȡ������ͨ�������Ϊ________��
��Al��Mg�Ͻ�ǰ��NaOH��Һ����Al2O3Ĥ���仯ѧ����ʽΪ____________________________________��
���ӹ�����ʹ�õı�����Ϊ________(�ѧʽ)��
(2)��ĸ�������Ϊ�Ͻ�֡�
�ٽ����ں�ˮ�з����ĵ绯ѧ��ʴ��ҪΪ________��
�ں�ĸ�øֿ��ɵ�����ұ�����ɣ���������������Ϊ���躬������������Ϊ________��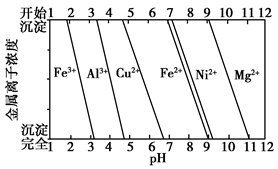
(3)��ĸ��������Ҫ��ͭ�Ͻ����졣
��80.0 g Cu��Al�Ͻ�������ȫ�ܽ���������ˮ�����˵ð�ɫ����39.0 g����Ͻ���Cu����������Ϊ________��
��Ϊ����ijͭ�Ͻ�ijɷ֣����Ὣ����ȫ�ܽ����NaOH��Һ����pH����pH��3.4ʱ��ʼ���ֳ������ֱ���pHΪ7.0��8.0ʱ���˳����������ͼ��Ϣ�ƶϸúϽ��г�ͭ��һ������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ҵ��������Ҫ�ɷ�ΪSiO2��Fe2O3��Al2O3��MgO���ָ������з���������д����Ի��շ����еĽ���Ԫ�ء�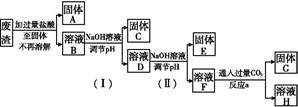
�ش��������⡣
(1)����A�Ļ�ѧʽΪ�������� ��
(2)��Ӧa�Ļ�ѧ��Ӧ����ʽΪ�� ��
(3)25 ��ʱ,��Һ�н������ӵ����ʵ���Ũ������ҺpH�Ĺ�ϵ��ͼ��ʾ: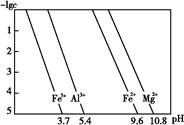
�ٵ���pH(��)��pHΪ������ ��;
����ҺD��������������Һ��ͬʱ����pH(��)��������Ӧ�����ӷ���ʽΪ�� ;
�۴�ͼ�����ݼ���ɵ�Mg(OH)2���ܶȻ�Ksp[Mg(OH)2]ԼΪ���� ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ú�������������ͭ��ȡ�Ȼ�ͭ����(CuCl2��xH2O)�������²���:
��֪:��pHΪ4��5ʱ,Fe3+������ȫˮ�������,����ʱCu2+ȴ������ˮ�⡣
(1)������A��ѡ����������(����,��ͬ)��
��Cl2 ��H2O2 ��HNO3 ��KMnO4
(2)Ҫ�õ��ϴ��IJ�Ʒ,�Լ�B��ѡ������������
��NaOH ��FeO ��CuO ��Cu2(OH)2CO3
(3)����Һ�����ᾧ�õ��Ȼ�ͭ�ķ�������������(��ʵ���Ⱥ�˳������)��
�ٹ��� ����ȴ ������Ũ�� ����������
(4)Ϊ�˲ⶨ�Ƶõ��Ȼ�ͭ����(CuCl2��xH2O)��xֵ,ij��ȤС�����������ʵ�鷽��:
����һ:��ȡm g�����������������ټ���Ϊֹ,��ȴ������������ˮCuCl2������Ϊn g��
������:��ȡm g����,��������10%������������Һ�����ˡ�ϴ�Ӻ���С��������������ټ���Ϊֹ,��ȴ���������ù��������Ϊn g��
�ٷ���һ������CuCl2��xH2O�õ���ˮCuCl2,Ӧ����������(����������)�н��С�
������������һ����10%������������Һ,����������Ǽ��㡢����������������
����������������ʵ�鷽��,���п��еķ�������������,��һ�ַ��������е������� ��,���÷�����,�����x=��������������(�ú�m��n�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ӻ���Ag����Fe3����Al3������Һ�У�����ͼ��ʾ����������ȡ��������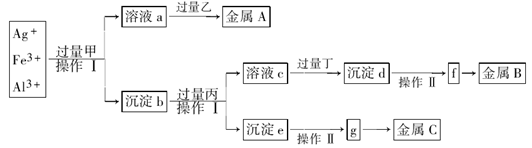
��֪��ʵ��ʱ�ס��ҡ���������������ֻ�ܴ�̼��李��������ơ�ϡ���ᡢ������������Һ��ѡ��Ҳ������������Һ����ȡ���Ը�ʵ����̵ķ�����ȷ����
| A������NaOH������ϡH2SO4 |
| B�����������ǣ������ҵĻ�ԭ�Խ�����A����Һa�л�ԭ���� |
| C������I�ǹ��˺�ϴ�ӳ�����ϴ�ӳ����ķ����ǽ�©���еij���ת�Ƶ�С�ձ��У�������ˮ��û�������ò��������裬Ȼ��ˮ�������ظ�2-3�� |
| D��g�����������c���õ�ⷨ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Co(OH)2�ڿ����м���ʱ��������������¶ȵı仯��ͼ��ʾ�� 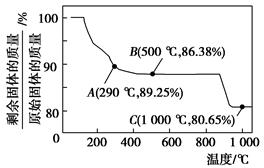
�ܵ��й��������£�Co(OH)2�������ԡ��ܵ���Ҫ��������ѧ�������������ӽ���������Ԫ�ء�
���������Ϣ�Իش��������⣺
(1)Co(OH)2���Ʊ�����CoCl2��Һ�м��Թ����İ�ˮ������NaOH��Һ��ԭ����________________________________(�û�ѧ����ʽ��ʾ)���Ƶõ�Co(OH)2�����ڿ����г��ڷ��ã��ᱻ�����е�O2��������ѧ����ʽΪ_____________________________________________________��
(2)��ͼ����֪�ܵ��������������290 ��ʱ����ȫ��ˮ����1000 ��ʱ��ʣ�����ijɷ�Ϊ________________________(�ѧʽ)����290��500 �淶Χ�ڣ������ķ�Ӧ�Ļ�ѧ����ʽΪ__________________________________________��
(3)��֪�����£���0.10 mol��L��1 CoCl2��Һ�м��백ˮ��ֽ��裬��Co(OH)2�������ɣ�����Һ��pH��8ʱ��c(Co2��)��________mol��L��1(Ksp[Co(OH)2]��1.6��10��15)�������·�ӦCo2����2H2O??Co(OH)2��2H����ƽ�ⳣ��Ϊ
_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ʯ����Ҫ�ɷ�ΪK2SO4��Al2(SO4)3��2Al2O3��6H2O�������������Fe2O3���ʡ�ijУ�о�С����������ʯ�Ʊ������������������£�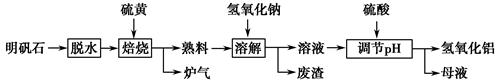
(1)�����ա������з�Ӧ�Ļ�ѧ����ʽΪ Al2(SO4)3��
Al2(SO4)3�� S
S
 Al2O3��
Al2O3�� ________����
________����
(2)������Һ�ͷ����IJ�����________�����ܽ⡱ʱ��Ӧ�����ӷ���ʽΪ_________________________________��
(3)������pH������ˡ�ϴ��Al(OH)3������֤��������ϴ�Ӹɾ���ʵ�������������________��
(4)��ĸҺ���пɻ��յ�������________��
(5)�������ա�������ǡ������48 g��ƣ������������տɵõ�________ g����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������Ļ�������;�㷺����ش��������⣺
��1��д���ڳ�ʪ�����и�����ʴʱ������Ӧ�ĵ缫��Ӧʽ��_______________________��
���������м����H2SO4��Һ�г���ܽ������Һ�в�δ����Fe3���������ӷ���ʽ˵��ԭ��______________________________________��
��2�����������صķ�ӦʽΪ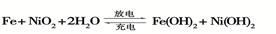
�ٰ��������ص������� ���õ�ع���һ��ʱ�������磬���ʱ����Fe�ķ�Ӧ������______��
�ڸ�������(Na2FeO4)��һ�����;�ˮ��������ͼװ�ÿ�����ȡ�����������ơ�д�����õ�ⷨ��ȡ��������ʱ�������ĵ缫��Ӧʽ___________________________________��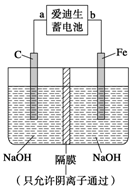
������Ϊ����������Ϊһ�����;�ˮ�������ɴ������______��
a��������������ˮ�γ�һ�ֽ��壬���н�ǿ��������
b����������������ɱ��ʱ����ԭ����Fe3����Fe3��ˮ�������������������������������
c���������ƾ���ǿ�����ԣ�������ɱ��
d���������ƾ���ǿ��ԭ�ԣ�������ɱ��
��3��ijFeO��Fe2O3������У������������ʵ���֮��Ϊ4�U5������Fe2+��Fe3+���ʵ���֮��Ϊ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��0��1mol��þ�����������100ml 2mol��L��H2SO4�У�Ȼ��μ�1mol��L��NaOH��Һ����ش�
��1�����ڵμ�NaOH��Һ�Ĺ����У����ɳ���������m�����NaOH��Һ�������ϵ��ͼ��ʾ����ش𣺵�V1=160mLʱ���������ĩ��n��Mg��=_____mol��V2=______mL��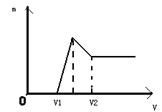
��2�����ڵμ�NaOH��Һ�Ĺ�������ʹMg2+��Al3+�պó�����ȫ�������NaOH��Һ���Ϊ________ml
��3�����������Ϊ0��1mol����þ�۵����ʵ����ķ���Ϊa����100ml2mol��L�������ܽ�˻������ټ���450ml1mol��L������������Һ�����ó�������Al��OH��3�����������a��ȡֵ��Χ��__ ___��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com