
�������Ļ�������;�㷺����ش��������⣺
��1��д���ڳ�ʪ�����и�����ʴʱ������Ӧ�ĵ缫��Ӧʽ��_______________________��
���������м����H2SO4��Һ�г���ܽ������Һ�в�δ����Fe3���������ӷ���ʽ˵��ԭ��______________________________________��
��2�����������صķ�ӦʽΪ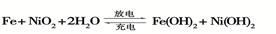
�ٰ��������ص������� ���õ�ع���һ��ʱ�������磬���ʱ����Fe�ķ�Ӧ������______��
�ڸ�������(Na2FeO4)��һ�����;�ˮ��������ͼװ�ÿ�����ȡ�����������ơ�д�����õ�ⷨ��ȡ��������ʱ�������ĵ缫��Ӧʽ___________________________________��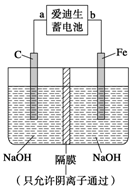
������Ϊ����������Ϊһ�����;�ˮ�������ɴ������______��
a��������������ˮ�γ�һ�ֽ��壬���н�ǿ��������
b����������������ɱ��ʱ����ԭ����Fe3����Fe3��ˮ�������������������������������
c���������ƾ���ǿ�����ԣ�������ɱ��
d���������ƾ���ǿ��ԭ�ԣ�������ɱ��
��3��ijFeO��Fe2O3������У������������ʵ���֮��Ϊ4�U5������Fe2+��Fe3+���ʵ���֮��Ϊ___________��
��1��O2+2H2O+4e-=4OH-��1�֣�;2Fe3++Fe=3Fe2+��1�֣� Fe2O3+6H+=2Fe3++3H2O����д���۷֣�
��2����NiO2��1�֣�;��ԭ��Ӧ��1�֣�
��Fe-6e-+8OH-=FeO42-+4H2O��2�֣�
��ad��2�֣�
��3��1��1��2�֣�
���������������1���ڳ�ʪ�����и�����ʴʱ����ΪO2��H2O����ʱ�õ�������OH?��O2+2H2O+4e-=4OH-��Fe��ԭ��Fe3+����ط�ӦΪ��Fe2O3+6H+=2Fe3++3H2O ��2Fe3++Fe=3Fe2+��
��2�������������õ��ӷ�Ӧ�����ݻ�ѧ����ʽ��֪����ΪNiO2�����ʱ������������Fe����Ӧ����Ϊ��ԭ��Ӧ��
��Fe������ʧ��������FeO42?���缫����ʽΪ��Fe-6e-+8OH-=FeO42-+4H2O
�۸������Ʊ���ԭΪFe3+��Fe3+ˮ������Fe(OH)3���壬���н�ǿ�����ԣ���a�����b����ȷ��������������Ԫ�ش��ڸ�̬������ǿ�����ԣ���ɱ����������c����ȷ��d�����
��3�����������������ʵ���֮��Ϊ4�U5���ɵ�[n(FeO)+2n(Fe2O3)]��[n(FeO)+3 n(Fe2O3)]=4:5���ɵ�n(FeO)��n(Fe2O3)=2����n(Fe2+)��n(Fe3+)=1:1��
���㣺���⿼��ԭ���ԭ��������ʽ����д�����ʵ����ʺ���;�������ļ��㡣


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ʯ����ȡ�طʺ�������������Ҫԭ�ϣ�����ʯ����ɺ�����[KAl(SO4)2?12H2O]���ƣ�����������������������������ʡ�����ʵ�鲽������ͼ��ʾ�� 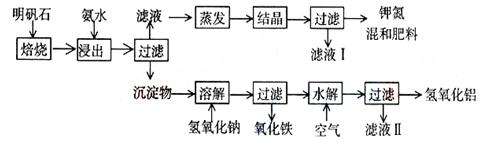
��������ͼʾ�����������գ�
��1������ʯ���պ���ϡ��ˮ����������500mlϡ��ˮ��ÿ������39.00g������ҪȡŨ��ˮ��ÿ������250g����__________ml���ù��Ϊ_______ml��Ͳ��ȡ��
��2����ˮ������õ���������ϵ�����ˣ���Һ�г�K����SO42���⣬���д�����NH4��������NH4���ķ�����_______________________________________________________��
��3��д�����������������ʵĻ�ѧʽ________________________________________��
��4����Һ�ijɷ���ˮ��______________��
��5��Ϊ�ⶨ��Ϸ���K2SO4��(NH4)2SO4�мصĺ��������������в��裺
�ٳ�ȡ�ص�������������ˮ����������______��Һ��������ɫ��������_______��______������(��ʵ���������)������ȴ�����ء�
��6��������Ϊmg�����������ʵ���Ϊn mol����������K2SO4�����ʵ���Ϊ��______���ú���m��n�Ĵ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ�У�A��B��C��D��E�ǵ��ʣ�G��H��I��F��B��C��D��E�ֱ��A�γɵĶ�Ԫ�������֪��
�ٷ�ӦC+G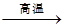 B+H�ܷų��������ȣ��÷�Ӧ��Ӧ��������ĺ��ӣ�
B+H�ܷų��������ȣ��÷�Ӧ��Ӧ��������ĺ��ӣ�
��I��һ�ֳ������������壬����E���Է�����Ӧ��2E+I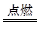 2F+D��F��EԪ�ص���������Ϊ60%��
2F+D��F��EԪ�ص���������Ϊ60%��
�ش����⣺
��1�����з�Ӧ�Ļ�ѧ����ʽΪ��________________________________________��
��2��1.6 g G�������ᣬ�õ�����Һ��ͭ����ȫ��Ӧ������������ͭ�۵�������д�����ӷ���ʽ�ͼ�����̣���__________________________________________��
��3��C�����NaOH��Һ��Ӧ�����ӷ���ʽΪ��___________________________����Ӧ����Һ�����������I��Ӧ�����ӷ���ʽΪ��_________________________��
��4��E��I��ȼ�չ۲쵽�������ǣ�____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ڽ������ӵ�ij�ֺ�������ҩƤ�ɴ���ʯ��ˮ�ࡢ���������ƶ��ɡ�
��1��Al��ԭ�ӽṹʾ��ͼΪ____________��Al��NaOH��Һ��Ӧ�����ӷ���ʽΪ____________��
��2��30Si��ԭ�ӵ�������Ϊ________________________��
��3��Al3+��Yn-�ĵ�������ͬ��Y�������Ԫ�ص��⻯���ˮ��Һ�������ԣ�������⻯���зе���͵���____________________________________��
��4�����ӹ����У�ҩƤ�ڸ����²�����������ʹ�����������������壬��������____________��
��5���������������36.0 g������Fe2O3��Al2O3��SiO2������������ϡ���ᣬ����õ�11.0 g���壻��Һ�м������NaOH��Һ������õ�21.4 g���壻���������Al2O3����������Ϊ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ӹ�ҵ����30%��FeCl3��Һ��ʴ���ھ�Ե���ϵ�ͭ��������ӡˢ��·�塣
��1��FeCl3��Һ�����ͭ������Ӧ�����ӷ���ʽ�� ��
��2��������Һ��Fe3+���ڵIJ�������������� ��
��3��ij����ʦΪ�˴�ʹ�ù��ĸ�ʴ��Һ�л���ͭ�������»���Ȼ�����Һ�����������в��裺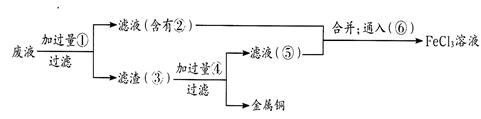
����ʵ���м�������ɵ��й����ʵĻ�ѧʽΪ�� ��____________
ͨ��õ�FeCl3��Һ�����ӷ�Ӧ����ʽΪ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��20 mL ijŨ�ȵ�AlCl3��Һ�е���2 mol��L��1��NaOH��Һʱ�����õij������������NaOH��Һ�����֮��Ĺ�ϵ��ͼ��ʾ��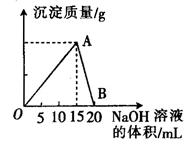
��1��ͼ��A���ʾ��������______________��
��2������������______________g��
��3��B���ʾ��������______________��
��4������AlCl3��Һ�����ʵ���Ũ����______________��
��5�������ó�����Ϊ0.39��ʱ����ȥNaOH��Һ�������_____ mL ��_______ mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ����ij����������Cu2O��Al2O3��Fe2O3��SiO2����ȡͭ�IJ����������£�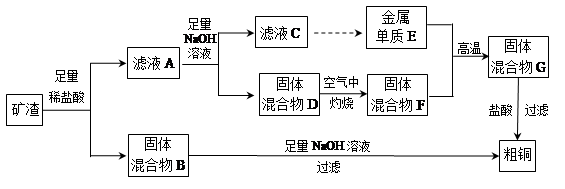
��֪��Cu2O+2H+�TCu+Cu2++H2O
��1����������B������������Һ��Ӧ�Ļ�ѧ����ʽΪ ��
��2����ҺA����Ԫ�صĴ�����ʽֻ��ΪFe2+��������
���漰�����ӷ���ʽΪ ��
��������дCu2O������ķ�Ӧ����������ҺA��Fe2+���Լ�Ϊ �����Լ����ƣ���
��3������ҺC�е���Ԫ���Գ�����ʽ��������ѡ�õ�����Լ�Ϊ __ ������ţ���
| A������������Һ | B��������Һ | C����ˮ | D��������̼ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ͭ������ϡ���ᣬ��������������Һ���ֽ�һ������ͭƬ���뵽100 mLϡ������������Ļ����Һ�У�ͭƬ��ȫ�ܽ⣨�������ε�ˮ�⼰��Һ����ı仯����
��1��д��ͭ�ܽ������������Һ�����ӷ���ʽ_______________________________________��
��2����ͭ��ȫ�ܽ�ʱ����Һ�е�Fe3����Cu2����H���������ӵ����ʵ���Ũ����ȣ��Ҳ����Һ��pH��1�����ܽ�ͭ��������_________g����Һ�е�c(SO42��)��__________mol/L��
��3����������ͼ��ʾ��װ���з�����1���еķ�Ӧ����X���� ���������������缫��Ӧʽ ��Y���IJ����� ���缫��Ӧʽ ��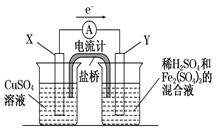
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ȸʯ��Ҫ��Cu2��OH��2CO3����������Fe��Si�Ļ����ʵ�����Կ�ȸʯΪԭ���Ʊ� CuSO4��5H2O�����ײ���G���������£�
��ش��������⣺
��1����ҺA�Ľ���������Cu2+��Fe2+��Fe3+�������������Լ���ѡ��ʵ�鲽�����Լ������Ϊ_____������ţ���
a��KMnO4 b����NH4��2S c��H2O2 d��KSCN
����ҺB�м���CuO�������� ��
��2������ҺC���CuSO4��5H2O����Ҫ���� ���� �����˵Ȳ��������ձ���©���⣬���˲������õ���һ�����������������ڴ˲����е���Ҫ������ ��������
��3��д���Ʊ�G�Ļ�ѧ��Ӧ����ʽ����ʵ��������а����ݳ���Ӧѡ����������������װ�û��գ�����ţ���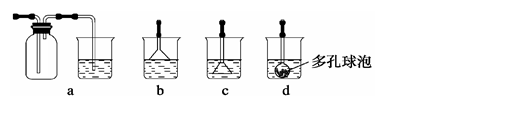
��4�����ⶨ��ҺA��Fe2+����Һ������KMnO4����Һ�ζ���ȡ��KMnO4����ҺӦʹ�� ��ȡA��Һϡ��һ����������KMnO4����Һ�ζ����յ�����Ϊ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com