
����ʯ����Ҫ�ɷ�ΪK2SO4��Al2(SO4)3��2Al2O3��6H2O�������������Fe2O3���ʡ�ijУ�о�С����������ʯ�Ʊ������������������£�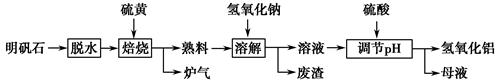
(1)�����ա������з�Ӧ�Ļ�ѧ����ʽΪ Al2(SO4)3��
Al2(SO4)3�� S
S
 Al2O3��
Al2O3�� ________����
________����
(2)������Һ�ͷ����IJ�����________�����ܽ⡱ʱ��Ӧ�����ӷ���ʽΪ_________________________________��
(3)������pH������ˡ�ϴ��Al(OH)3������֤��������ϴ�Ӹɾ���ʵ�������������________��
(4)��ĸҺ���пɻ��յ�������________��
(5)�������ա�������ǡ������48 g��ƣ������������տɵõ�________ g����������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����A���ĩ������B��ɵĻ�����ܷ�����ͼ��ʾ��һϵ�з�Ӧ��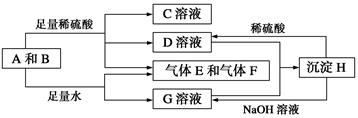
��ش��������⣺
(1)���A���ʵ�Ԫ�������ڱ��д��ڵ�__________����__________�塣
(2)������B�ĵ���ʽΪ________________________��
(3)D��G����Һ��Ϻ�����Ӧ�����ӷ���ʽΪ______________________________
(4)�����£�D��Һ��pH________7(�����������������)����ԭ����____________________________(�����ӷ���ʽ��ʾ)��
(5)10.8 g A������������NaOH��Һ��Ӧ������������������Ϊ________ g��
(6)��̼����ϡ���ᡢ����E������F���ȼ�ϵ�أ��õ�ص�������ӦʽΪ______________________���Ըõ��Ϊ��Դ���ö��Ե缫���100 g 8%��C��Һ�������ʵ���������Ϊ12.5%ʱֹͣ��⣬��������У����ɵ�������״���µ������Ϊ________ L����·��ͨ�����ӵ����ʵ���Ϊ________ mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����£�����A��B��C�ֱ�Ϊ���塢����ɫ���塢��ɫ���塣�ں��ʵķ�Ӧ�����£����ǿ��������ͼ���з�Ӧ����֪E��Һ����ɫ�ġ���ش�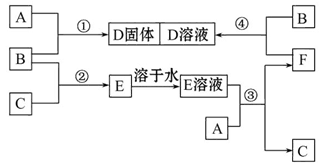
��1��A��_______��B��_______��C��_______ �����ѧʽ����
��2����Ӧ�ٵĻ�ѧ����ʽΪ�� ___________________________________��
��3����Ӧ�۵����ӷ���ʽΪ�� _________________________________________��
��4����Ӧ�ܵ����ӷ���ʽΪ��___________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ�У�A��B��C��D��E�ǵ��ʣ�G��H��I��F��B��C��D��E�ֱ��A�γɵĶ�Ԫ�������֪��
�ٷ�ӦC+G B+H�ܷų��������ȣ��÷�Ӧ��Ӧ��������ĺ��ӣ�
B+H�ܷų��������ȣ��÷�Ӧ��Ӧ��������ĺ��ӣ�
��I��һ�ֳ������������壬����E���Է�����Ӧ��2E+I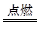 2F+D��F��EԪ�ص���������Ϊ60%��
2F+D��F��EԪ�ص���������Ϊ60%��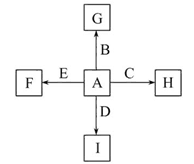
�ش����⣺
��1�����з�Ӧ�Ļ�ѧ����ʽΪ��________________________________________��
��2��1.6 g G�������ᣬ�õ�����Һ��ͭ����ȫ��Ӧ������������ͭ�۵�������д�����ӷ���ʽ�ͼ�����̣���__________________________________________��
��3��C�����NaOH��Һ��Ӧ�����ӷ���ʽΪ��___________________________����Ӧ����Һ�����������I��Ӧ�����ӷ���ʽΪ��_________________________��
��4��E��I��ȼ�չ۲쵽�������ǣ�____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾ����һЩ�����ĵ��ʡ�������֮���ת����ϵͼ����Щ��Ӧ�еIJ������ʺͷ�Ӧ��������ȥ��������A�Ǽ�ͥ�����г��õ�һ�ֵ�ζƷ����Ӧ�ٳ�֮Ϊ���ȼҵ����D�ǻ���ɫ���壬H��ˮ��Һ����Ư�ס�ɱ���ԣ�JΪ���ɫ������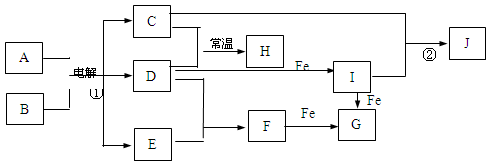
��1��H�Ļ�ѧʽΪ ��
��2��д��I��Fe��Ӧ����G�����ӷ���ʽΪ ��
��3����Ӧ�ٵĻ�ѧ����ʽΪ ��
��4����Ӧ�ڵ����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ڽ������ӵ�ij�ֺ�������ҩƤ�ɴ���ʯ��ˮ�ࡢ���������ƶ��ɡ�
��1��Al��ԭ�ӽṹʾ��ͼΪ____________��Al��NaOH��Һ��Ӧ�����ӷ���ʽΪ____________��
��2��30Si��ԭ�ӵ�������Ϊ________________________��
��3��Al3+��Yn-�ĵ�������ͬ��Y�������Ԫ�ص��⻯���ˮ��Һ�������ԣ�������⻯���зе���͵���____________________________________��
��4�����ӹ����У�ҩƤ�ڸ����²�����������ʹ�����������������壬��������____________��
��5���������������36.0 g������Fe2O3��Al2O3��SiO2������������ϡ���ᣬ����õ�11.0 g���壻��Һ�м������NaOH��Һ������õ�21.4 g���壻���������Al2O3����������Ϊ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ӹ�ҵ����30%��FeCl3��Һ��ʴ���ھ�Ե���ϵ�ͭ��������ӡˢ��·�塣
��1��FeCl3��Һ�����ͭ������Ӧ�����ӷ���ʽ�� ��
��2��������Һ��Fe3+���ڵIJ�������������� ��
��3��ij����ʦΪ�˴�ʹ�ù��ĸ�ʴ��Һ�л���ͭ�������»���Ȼ�����Һ�����������в��裺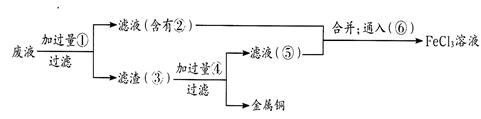
����ʵ���м�������ɵ��й����ʵĻ�ѧʽΪ�� ��____________
ͨ��õ�FeCl3��Һ�����ӷ�Ӧ����ʽΪ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ����ij����������Cu2O��Al2O3��Fe2O3��SiO2����ȡͭ�IJ����������£�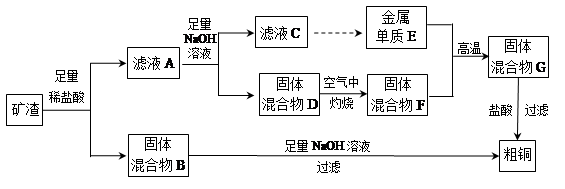
��֪��Cu2O+2H+�TCu+Cu2++H2O
��1����������B������������Һ��Ӧ�Ļ�ѧ����ʽΪ ��
��2����ҺA����Ԫ�صĴ�����ʽֻ��ΪFe2+��������
���漰�����ӷ���ʽΪ ��
��������дCu2O������ķ�Ӧ����������ҺA��Fe2+���Լ�Ϊ �����Լ����ƣ���
��3������ҺC�е���Ԫ���Գ�����ʽ��������ѡ�õ�����Լ�Ϊ __ ������ţ���
| A������������Һ | B��������Һ | C����ˮ | D��������̼ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������Ҫ�ɷ���Al2O3����SiO2��Fe2O3��MgO�����ʣ�����ȡ���ֹ��յ��������£�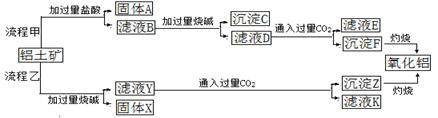
��ش��������⣺
��1�����̼������������Al3+�ķ���ʽΪ____________________________.
��2�������Ҽ����ռ������SiO32�������ӷ���ʽΪ______________________.
��3����֤��ҺB��Fe3+����ȡ������Һ������________�����Լ����ƣ���
��4����ҺE��K�����ʵ���Ҫ�ɷ���____________(�ѧʽ)��д������Һ��һ����;____________
��5����֪298Kʱ��Mg(OH)2���ܶȻ�����KSP=5.6��10��12��ȡ��������ҺB������һ�������ռ�ﵽ������Һƽ�⣬���PH=13.00������¶��²�������Һ�е�c(Mg2+)=__________________.
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com