
(10��) Na2S2O3?5H2O���׳ƺ�����������ҵ���õ�һ�ֶ�Ӱ������ҵ���Ƶõ�Na2S2O3?5H2O�����п��ܺ���Na2SO3��Ϊ�ⶨij������Ʒ�ijɷ֣���ȡ����������ͬ�ĸ���Ʒ���ֱ������ͬŨ�ȵ�������Һ30 mL����ַ�Ӧ��Na2S2O3��H2SO4=Na2SO4��SO2����S����H2O�����˳�������ҺʹSO2ȫ���ݳ�������й�ʵ���������±�����������ѻ���Ϊ��״������
| ��һ�� | �ڶ��� | ������ |
��Ʒ������/g | 6.830 | 13.660 | 30.000 |
����������������/L | 0.672 | 1.344 | 2.688 |
�������/g | 0.800 | 1.600 | 3.200 |
�Լ��㣺
��1������������Һ�����ʵ���Ũ��Ϊ ��
��2����Ʒ��n(Na2S2O3?5H2O)��n(Na2SO3)= ��
��3��ij�������С��������������Ʒ���ƺ�Na2S2O3 0.100 mol?L-1�ĺ�����Һ�����������ⶨij������ˮ��Ba2+��Ũ�ȡ�����ȡ��ˮ50.00 mL�������ʵ�����ȼ���������K2Cr2O7��Һ����BaCrO4������������ϴ�ӡ����˺���������ϡ�����ܽ⣬��ʱCrO42-ȫ��ת��ΪCr2O72-���ټӹ���KI��Һ����ַ�Ӧ�����������Ƶĺ�����Һ���еζ�����Ӧ��ȫʱ��������ĺ�����Һ�����Ϊ36.00 mL����֪�йط�Ӧ�����ӷ���ʽΪ��
��Cr2O72- + 6I- + 14H+ 2Cr3+ + 3I2 + 7H2O
��I2 + 2S2O32- 2I- + S4O62-
��I2 + SO32- + H2O 2I- + SO42- + 2H+
��ζ������п��� ��ָʾ��������ù�����ˮ��Ba2+�����ʵ���Ũ�ȡ�
��1��4 mol?L-1��2�֣�
��2��5��1 ��3�֣�
��3������ ��1�֣�
�⣺�ɣ�2��֪��n(Na2S2O3) = 0.036L��0.100 mol?L-1 = 0.0036mol
��n(Na2SO3) = 0.0036mol��5 = 0.00072mol
�������⣺��2BaCrO4 ~ Cr2O72- ~ 3I2 ~ 6S2O32-
n1(BaCrO4) = ![]() =
=![]() = 0.0012mol��1�֣�
= 0.0012mol��1�֣�
��2BaCrO4 ~ Cr2O72- ~ 3I2 ~ 3SO32-
n2(BaCrO4) = ![]() =
=![]() = 0.00048mol��1�֣�
= 0.00048mol��1�֣�
��c(Ba2+) = ![]() = 3.36��10-2 mol?L-1 ��2�֣�
= 3.36��10-2 mol?L-1 ��2�֣�


 53���ò�ϵ�д�
53���ò�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012����У�Ϻ��У�����������ʦ������У�����������ѧ�Ծ����������� ���ͣ�������
�����10�֣�
����������Ƥ�����Ƶ���Ҫ��ѧ�Լ���������ˮâ����Na2SO4����̿���ڸ����·�Ӧ���Ƶã���Ӧʽ���£�Na2SO4+ 4C Na2S+4CO�� �� Na2SO4+4CO
Na2S+4CO�� �� Na2SO4+4CO Na2S+4CO2
Na2S+4CO2
��1����Ҫ��ȡNa2S 7.80 g����������������ˮâ����Na2SO4����������Ϊ90%������������Ҫ��ˮâ����Na2SO4�� g����ȷ��0.01����
��2�����ڷ�Ӧ�����ɵ�Na2S���ʵ���Ϊ1 mol�������ĵ�̼���ʵ����ʵ���n�ķ�Χ��
�� n �� ��
��3������������Ӧ�����ĵ�̼����Ϊ1 mol������Na2S�����ʵ���Ϊy mol�����ɵ�CO��CO2�����ʵ���֮��Ϊx����y��x�Ĺ�ϵʽΪy= ��
��4��Na2S�����ڿ����У��ᱻ����������Na2SO3��Na2SO4���ֳ�ȡ�Ѿ�����������������Ʒ39.20 g����ˮ�У������������ᣬ��ַ�Ӧ����˵ó���9.6 g���ų�H2S����1.12 L����״����������㣺39.20 g��Ʒ�и�������������ʵ�����������������ˮ�е��ܽ⣩��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ����һ�и���10���¿���ѧ�Ծ����������� ���ͣ������
(��10��)���ֽⷴӦ����ѧ��ѧ�г�����һ�ַ�Ӧ���͡�
(1)��֪�ڳ����²��Ũ�Ⱦ�Ϊ0.1 mol��L��1������6����Һ��pHֵ��
| ���� | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN | C6H5ONa |
| pH | 8.8 | 9.7 | 11.6 | 10.3 | 11.1 | 11.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ����һ�и�����ѧ����ĩ��⻯ѧ�Ծ� ���ͣ������
(10��)�������������������ܽ�Ȳ�ͬ����˿���������һ���ʣ�������Һ��pH���ﵽ����������ӵ�Ŀ�ġ����ܽ��������������ڲ�ͬpH�µ��ܽ��(s��mol��L��1)����ͼ��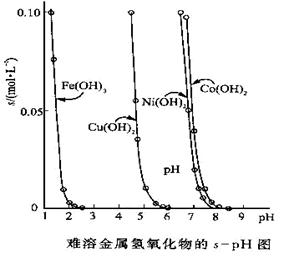
(1)pH��3ʱ��Һ��ͭԪ�ص���Ҫ������ʽ�ǣ�________(��ѧʽ)
(2)��Ҫ��ȥCuCl2��Һ�е�����Fe3����Ӧ�ÿ�����Һ��pHΪ
A����1 B��4���� C����6
(3)��Ni(NO3)2��Һ�к���������Co2�����ʣ�___ (�ܡ�����)ͨ��������ҺpH�ķ�������ȥ��������_________��
(4)��֪һЩ��������ܶȻ��������±���
| ���� | FeS | MnS | CuS | PbS | HgS | ZnS |
| Ksp | 6.3��10��18 | 2.5��10��13 | 1.3��10��36 | 3.4��10��28 | 6.4��10��53 | 1.6��10��24 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���㽭ʡ����10���¿���ѧ�Ծ��������棩 ���ͣ������
(��10��)���ֽⷴӦ����ѧ��ѧ�г�����һ�ַ�Ӧ���͡�
(1)��֪�ڳ����²��Ũ�Ⱦ�Ϊ0.1 mol��L��1������6����Һ��pHֵ��
|
���� |
CH3COONa |
NaHCO3 |
Na2CO3 |
NaClO |
NaCN |
C6H5ONa |
|
pH |
8.8 |
9.7 |
11.6 |
10.3 |
11.1 |
11.3 |
���ֽ��������һ�����ɣ�һ�ֽ�ǿ������һ�ֽ�������ο����Է��ط�Ӧ�����ɽ�����ͽ�ǿ����Σ��磺2CH3COOH+Na2CO3===2CH3COONa+CO2��+H2O�����ոù��ɣ����ж����з�Ӧ���ܳ�������______�����ţ���
A��CO2+H2O +2NaClO===Na2CO3+2HClO����������B��CH3COOH+NaCN===CH3COONa+HCN
C��CO2 +H2O +C6H5ONa��NaHCO3+C6H5OH ����D��CO2 +H2O +2C6H5ONa��Na2CO3+2C6H5OH
��2������ǰ����Ϣ�жϣ������£�Ũ�Ⱦ�Ϊ0.05 mol��L��1������5�����ʵ���Һ�У�pH��С���� (����)����pHֵԼΪ_______(����ֵ)��
��HCN ��CH3COOH ��HClO4 ��HClO ��H2SO4
(3)һЩ���ֽⷴӦ�ķ�������ѭ�����Ĺ��ɡ�����ת�������ڸ��ֽⷴӦ��
�ٹ�ҵ�Ͻ�ʯ�����봿����Һ��Ͽ��Ƶÿ�������Һ���ں����Ƽ�У���̼�������Һ�м��뱥��ʳ��ˮ�ɻ��С�մ��塣
�����������Ӧ���ܽ�����ֽⷴӦ��������һ���� ����������ۣ��ֽ�Na2S��AgI�����Ͻ��裬��Ӧ�����ӷ���ʽ ������������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ��һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��10��)����ʵ����ijͬѧ��ͬ����Ԫ�����ʵݱ����ʵ��ʱ���Լ������һ��ʵ�鷽��������¼���й�ʵ������(���±������еġ�ʵ�鷽�����롰ʵ������ǰ��һ���Ƕ�Ӧ��ϵ)��
|
ʵ�鲽�� |
ʵ������ |
|
�ٽ�þ����ɰֽ��ĥ�����Թ��У���������ˮ������ˮ���ڣ�������Һ�еμӷ�̪��Һ |
A������ˮ���ϣ��۳�С���Ĵ��ζ���������˻˻��������֮��ʧ����Һ��ɺ�ɫ�� |
|
�������Ƶõ�Na2S��Һ���������Ƶ���ˮ |
B���������������Һ���dz��ɫ |
|
�۽�һС������Ʒ�����з�̪��Һ����ˮ�� |
C�����ҷ�Ӧ��Ѹ�ٲ���������ɫ���壮 |
|
�ܽ�þ��Ͷ��ϡ������ |
D����Ӧ��ʮ�־��ң�������ɫ���塣 |
|
�ݽ�����Ͷ��ϡ������ |
E�����ɰ�ɫ��״�������̶�������ʧ |
|
����A1Cl3��Һ�еμ�NaOH��Һ������ |
F�����ɵ���ɫ������ |
���������ͬѧ���������ʵ�鱨�档
(1)ʵ��Ŀ�ģ��о� Ԫ�����ʵݱ���ɡ�
(2)ʵ����Ʒ���Լ��������ƣ�þ����������ϡ���ᣬ������ˮ������Na2S��Һ��AlC13��Һ��NaOH��Һ����̪��Һ�ȡ��������� ���� ���Թܣ��ԹܼУ���ͷ�ιܣ����ӣ�С��������Ƭ��ɰֽ�����ȡ�
(3)ʵ�����ݣ�(��д��ʵ�鲽���Ӧʵ������ı�ź͢ڵĻ�ѧ����ʽ����ʵ��Ľ���)
|
ʵ������ |
�� |
�� |
�� |
�� |
�� |
�� |
|
ʵ��������A~F�� |
|
|
|
|
|
|
�� ��
�� ��
��ʵ��Ľ��ۣ� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com