
����Ŀ����ҡͷ�����Ƕ�Ʒ�е�һ�֣����к��й��Ҽ�ܵ�ҩƷ�����Ȱ�ͪ�������»����ã���һ�������·���һϵ��ת����
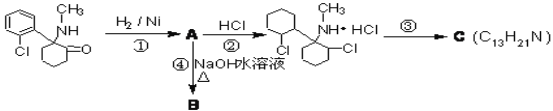
(1)�Ȱ�ͪ�к�������������Ϊ__________������ת�������з����ӳɷ�Ӧ����_____(�����)
(2)��Ӧ�۵�����__________��C�Ľṹ�����֣�����һ�ֽṹֻ����һ������̼ԭ��(�����ĸ���ͬԭ�ӻ�ԭ���ŵ�̼ԭ�ӽ�����̼ԭ��)����ṹ��ʽΪ__________
(3)����ܵĻ�ѧ��Ӧ����ʽ_____________________________________________
(4)��һ��ȥ���Ȱ�ͪD���ܷ������·�Ӧ��(��֪������ȩ��ķ���ʽΪC4H6O3)

����E��F��ת�������У�������⻯�������ֵ�����Ϊ_____________________
��H�ķ���ʽΪ_______________________
��M�ķ���ʽΪC10H10O3 ����������������ͬ���칹����___________��
i������ȩ�������ͬ������ ii�������ұ�����������ȡ����
��N��������ȩ�������ͬ������һ��ͬ���칹�壬��NΪ��Ҫԭ�Ϻϳ� ___________
___________

���𰸡�ͪ�����ʻ� �� ���������Ҵ���Һ������ 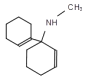
 + NaOH
+ NaOH![]()
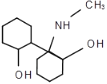 + NaCl�� ��ԭ�ԣ���ԭ���� C4H5O3N 18 OHCCH(CH3)COOH��HOCH2CH(CH3)COOH��BrCH2CH(CH3)COOH��CH2=C(CH3)COONa
+ NaCl�� ��ԭ�ԣ���ԭ���� C4H5O3N 18 OHCCH(CH3)COOH��HOCH2CH(CH3)COOH��BrCH2CH(CH3)COOH��CH2=C(CH3)COONa
��������
���ݺϳ�·�߿�֪��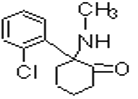 ��Ni������ʱ�����������ӳɷ�Ӧ����A�����Ƴ�AΪ
��Ni������ʱ�����������ӳɷ�Ӧ����A�����Ƴ�AΪ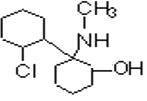 ��A��NaOHˮ��Һ���ȵ������»ᷢ��ˮ������
��A��NaOHˮ��Һ���ȵ������»ᷢ��ˮ������ ������BΪ
������BΪ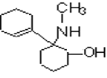 ��A��HCl����ȡ����Ӧ����
��A��HCl����ȡ����Ӧ����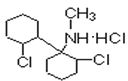 ���÷����ٷ�����ȥ��Ӧ����C���ݴ˷�������
���÷����ٷ�����ȥ��Ӧ����C���ݴ˷�������
��1���Ȱ�ͪ�Ľṹ��ʽΪ�� �����к�������������Ϊͪ�����ʻ����������ڼӳɷ�Ӧ��������
�����к�������������Ϊͪ�����ʻ����������ڼӳɷ�Ӧ��������
�ʴ�Ϊ��ͪ�����ʻ�������
��2����Ӧ��Ϊ ������ȥ��Ӧ�Ĺ��̣��䷴Ӧ����Ϊ�����������Ҵ���Һ�����ȣ��õ���C�����ֽṹ���ֱ��ǣ�
������ȥ��Ӧ�Ĺ��̣��䷴Ӧ����Ϊ�����������Ҵ���Һ�����ȣ��õ���C�����ֽṹ���ֱ��ǣ� ��
��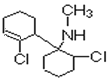 ����ֻ����һ������̼ԭ�ӣ���ṹ��ʽΪ��
����ֻ����һ������̼ԭ�ӣ���ṹ��ʽΪ�� ��
��
�ʴ�Ϊ�����������Ҵ���Һ�����ȣ� ��
��
��3��������Ϊ�л�������ԭ�ӷ���ˮ�ⱻȡ���Ĺ��̣��仯ѧ��Ӧ����ʽΪ�� + NaOH
+ NaOH![]()
 + NaCl��
+ NaCl��
�ʴ�Ϊ�� + NaOH
+ NaOH![]()
 + NaCl��
+ NaCl��
��4����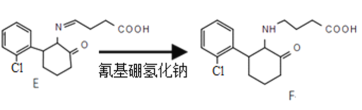 ̼��˫�������ԭ������������⻯�������ֵ�����Ϊ��ԭ�ԣ���ԭ������
̼��˫�������ԭ������������⻯�������ֵ�����Ϊ��ԭ�ԣ���ԭ������
�ʴ�Ϊ����ԭ�ԣ���ԭ������
������F![]() G�Ľṹ�仯��֪���ù��̷�����������ȡ������Ӧ��H�Ľṹ��ʽΪ��
G�Ľṹ�仯��֪���ù��̷�����������ȡ������Ӧ��H�Ľṹ��ʽΪ��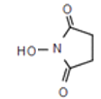 �����������ʽΪ��C4H5O3N��
�����������ʽΪ��C4H5O3N��
�ʴ�Ϊ��C4H5O3N��
�� M�ķ���ʽΪC10H10O3�����Ͷ� = ![]() = 6��������ȩ�������ͬ�����ţ���ȩ�����Ȼ����ֺ������ұ�����������ȡ������������ȡ�����IJ�ͬ����ɷ�6����ϣ��ֱ�Ϊ��-CHO��-CH2CH2COOH��-CHO��
= 6��������ȩ�������ͬ�����ţ���ȩ�����Ȼ����ֺ������ұ�����������ȡ������������ȡ�����IJ�ͬ����ɷ�6����ϣ��ֱ�Ϊ��-CHO��-CH2CH2COOH��-CHO��![]() ��- CH2CHO��- CH2 COOH��-CH2CH2CHO��-COOH��
��- CH2CHO��- CH2 COOH��-CH2CH2CHO��-COOH��![]() ��-COOH�Լ�
��-COOH�Լ�![]() ��- CH3���ٸ���ȡ������λ�÷�Ϊ�ڼ�����֣���֪�ܵ�����Ϊ6��3 = 18�֣�
��- CH3���ٸ���ȡ������λ�÷�Ϊ�ڼ�����֣���֪�ܵ�����Ϊ6��3 = 18�֣�
�ʴ�Ϊ��18��
��N��������ȩ�������ͬ������һ��ͬ���칹�壬��NӦΪOHCCH(CH3)COOH�����ݷ�Ӧ����������ϳɷ�������֪�����ĸ�������Ӧ��д![]() ���м�����ΪHBr����ӦΪ�ǻ���ȡ����Ӧ�������ٽ�����ԭ�ӵ���ȥ���̵õ�̼̼˫�������Եڶ�������ӦΪȩ������ԭΪ�ǻ��Ĺ��̣������ϳ�·�����£�
���м�����ΪHBr����ӦΪ�ǻ���ȡ����Ӧ�������ٽ�����ԭ�ӵ���ȥ���̵õ�̼̼˫�������Եڶ�������ӦΪȩ������ԭΪ�ǻ��Ĺ��̣������ϳ�·�����£�
 ��
��
�ʴ�Ϊ��OHCCH(CH3)COOH��HOCH2CH(CH3)COOH��BrCH2CH(CH3)COOH��CH2=C(CH3)COONa��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ϳ�״CaCO3��Ӧʱ������ʹ��Ӧ������������Լӿ����
A. ���������������һ��B. �����Ũ������һ������������
C. �¶�����30 ��D. ���ø�С���CaCO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ƿ��������ʪ�Թؽ��ס���������֢�����ף���ϳ�·�����£�

��֪��i��![]()
ii��![]()
iii��
������R��![]() ����������
����������
(1)A���ڷ���������ṹ��ʽ��_______________��
(2)B�Ľṹ��ʽ��__________��D�к��й����ŵ�������_____________��
(3)E����ϩ����E��B2��Ӧ����F�Ļ�ѧ����ʽ��____________________________��
(4)��Ӧ�ٵĻ�ѧ����ʽ��_________________����Ӧ�ڵķ�Ӧ������______________��
(5)��J��NaOH��Һ��Ͻ�����ټ����Ҵ��д�����ɫ����K��������K�Ľṹ��ʽ��____________��
(6)�� ��Ϊͬ���칹�壬�ҷ�������Ҫ��Ľṹ����_______�֡�
��Ϊͬ���칹�壬�ҷ�������Ҫ��Ľṹ����_______�֡�
�ٽṹ�к��б������ұ�����ֻ������ȡ����
������NaOH��Һ�з���ˮ�ⷴӦ
���ܺ�������Һ��������
(7)����ϩΪ��ʼԭ�ϣ������֪��Ϣѡ�ñ�Ҫ�����Լ��ϳɾ۶������Ҷ�����(  )��д��������5���ĺϳ�·��(�ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ����)��_____________________
)��д��������5���ĺϳ�·��(�ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ����)��_____________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ����˲ⶨ����Ħ�������̽��ʵ�飬��������طֽ���O2��ʵ�鲽�����£������Ӻ�ʵ��װ�ã����װ�õ������ԡ���������������ط�ĩ�������������̷�ĩ��Ͼ��ȣ����������Թ��У�ȷ�����Թܺ�ҩƷ��������Ϊ15.95 g�������ȣ���ʼ��Ӧ��ֱ���������������Ϊֹ����������Ͳ��ˮ�����Ϊ285.0 mL������ɱ�״�������������Ϊ278.8 mL����ȷ�����ԹܺͲ����������Ϊ15. 55 g��

��������ʵ����̣��ش��������⣺
��1����μ��װ�õ������ԣ�________��
��2�����в������ʵ�����ʱ�������Ӷ��������ȡ���������_____������ƫ������ƫС��������Ӱ��������
��3��ʵ������в������������ʵ�����__mol������С�������λ����ʵ��������������Ħ�������__������С�������λ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��1molij��������8molCl2��ȫȡ������������ķ���ʽΪ( )
A.CH4B.C2H6C.C3H8D.C4H10
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʳƷ�㾫������������·�ߣ���Ӧ������ȥ�����£�

��������������ǣ� ��
A�����裨1�������в����ı��ӿ���FeCl3��Һ����
B�����ӺͲ���������������KMnO4��Һ������Ӧ
C����������Ͳ�����������NaOH��Һ������Ӧ
D�����裨2�������в����ı�ϩ��������ˮ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E��F��G��H��I��J ��Ϊ�л�������������¿�ͼ���ش����⣺

��1��B��C��Ϊ��֧�����л������B�Ľṹ��ʽΪ_______________��C��Ũ���������¼��ȷ�Ӧֻ������һ��ϩ��D��D�Ľṹ��ʽΪ��_____________________��
��2��G�ܷ���������Ӧ��Ҳ��ʹ������Ȼ�̼��Һ��ɫ����G�Ľṹ��ʽΪ___________________________________________��
��3�����Ļ�ѧ����ʽ��________________________________________________��
���Ļ�ѧ����ʽ��___________________________________________________��
��4�����ķ�Ӧ������________________�����ķ�Ӧ������________________�� ���ķ�Ӧ������_____________________��
��5����H������ͬ�����ŵ�H��ͬ���칹��Ľṹ��ʽΪ____________________
______________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij˫ԭ�ӷ��ӹ��ɵ����壬��Ħ������ΪM g/mol������������Ϊm g�������ӵ�����ΪNA����
��1������������ʵ���Ϊ_______________________��
��2���������ڱ�״���µ����Ϊ______________L��
��3���������ڱ�״���µ��ܶ�Ϊ____________g/L��
��4������������ԭ������Ϊ___________________����
��5���������һ�����ӵ�����Ϊ_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾһЩ�����е�ijЩ�ṹ����ش��������⣺

(1)�������ʯ����(������ĸ����ͬ)________������ÿ��̼ԭ����________��̼ԭ������Ҿ�����ȡ����ʯ����________���塣
(2)����ʯī����________��ÿ����������ռ�е�̼ԭ����ƽ��Ϊ________����
(3)����NaCl����________��ÿ��Na����Χ��������Ҿ�����ȵ�Na����________����
(4)����CsCl����________��������________���壬ÿ��Cs����________��Cl�����ڡ�
(5)�����ɱ�����________��������________���壬ÿ��CO2������________��CO2���ӽ��ڡ�
(6)��֪ʯī��̼̼���ļ����Ƚ��ʯ��̼̼���ļ����̣����������������۵��ɸߵ��͵�����˳��Ϊ______________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com