
ijͬѧ����ԭ���ԭ����ʵ��ʱ,������ʵ�鲽��:
���õ��߽����������Ƶ����˷ֱ��봿����пƬ��ͭƬ������(��ͼ1);
�ڰ�һ�鴿����пƬ����ʢ��ϡ������ձ���;
�۰�һ�鴿����ͭƬ����ʢ��ϡ������ձ���;
���õ��߰�пƬ��ͭƬ����������,��ƽ�еز���ʢ��ϡ������ձ���(��ͼ2)��
�ش���������:
(1)ʵ�鲽�����Ӧ�۲쵽���������� ��
(2)ʵ�鲽�����Ӧ�۲쵽���������� ��
(3)ʵ�鲽�����Ӧ�۲쵽���������� ��
(4)ʵ�鲽�����Ӧ�۲쵽���������� ��
(5)ͨ��ʵ�鲽��ܸ�ͬѧͷ��������һ������(�����),�ò���������
(6)Ϊ��֤ʵ�ò���,��ͬѧ������˵ڢݲ�ʵ��,���Ҫ�����ڢݲ�ʵ���װ��ʾ��ͼ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��6��)ZnMnO2�ɵ��Ӧ�ù㷺����������Һ��ZnCl2NH4Cl�����Һ��
��1���õ�صĸ���������________����ع���ʱ����������________(��������� �� )��
��2����ZnCl2NH4Cl�����Һ�к�������Cu2���������ij�缫�ĸ�ʴ������Ҫԭ����____________��
����ȥCu2�������ѡ�������Լ��е�________(�����)��
| A��NaOH | B��Zn | C��Fe | D��NH3��H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
O3���ɳ���������(ԭ����ͼ)���ϡ�����Ƶá�
(1)ͼ������Ϊ (�A����B��)����缫��ӦʽΪ ��
(2)��C��ͨ��O2����A���ĵ缫��ӦʽΪ ��
(3)��C����ͨ��O2��D��E���ֱ��ռ���x L��y L����(��״��)����E���ռ���������O3��ռ���������Ϊ (����O3�ķֽ�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ��ˮ�г�����һ������Cr2O72-��CrO42-�����ǻ�����༰��̬ϵͳ�����ܴ���������д��������õĴ�������֮һ�ǵ�ⷨ��
(1)�÷���Fe���缫��⺬Cr2O72-�����Է�ˮ�����ŵ����У�������������ҺpH���ߣ�����Cr(OH)3��������Fe���缫��ԭ��Ϊ ��
(2)������������ҺpH���ߵ�ԭ����(�õ缫��Ӧ����) ����Һ��ͬʱ���ɵij������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ȣ�ClO2����һ�ֻ���ɫ�д̼�����ζ�����壬���۵�Ϊ��59�棬�е�Ϊ11.0�棬������ˮ����ҵ�����Գ�ʪ��KClO3�Ͳ��ᣨH2C2O4����60��ʱ��Ӧ�Ƶá�ijѧ��������ͼ��ʾװ��ģ�ҵ��ȡ���ռ�ClO2��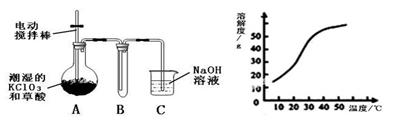
��1��A���������¶ȿ���װ�ã����ƾ��ơ��¶ȼ��⣬����Ҫ�IJ��������� ��
��2����Ӧ����װ��C�пɵ�NaClO2��Һ����֪���¶ȵ���38��ʱNaClO2������Һ������������NaClO2��3H2O�����¶ȸ���38��ʱ����������NaClO2����������ͼ��ʾ��NaClO2���ܽ�����ߣ��벹���NaClO2��Һ���Ƶ�NaClO2����IJ������裺 �� �����ᾧ���� ���� ϴ�ӣ��� ���
��3��ClO2�ܲ��ȶ������������ƣ���ˮ���յõ�ClO2��Һ��Ϊ�ⶨ������Һ��ClO2��Ũ�ȣ�����������ʵ�飺�� ȷ��ȡClO2��ҺV1mL���뵽��ƿ�У�����������ˮϡ�ͣ�����������pH��2.0���� ����������KI���壬����Ƭ�̡���ʱ������Ӧ�����ӷ���ʽΪ�� ���� �������ָʾ������c mol/L Na2S2O3��Һ�ζ������յ�ʱ����Na2S2O3��ҺV2 mL����ԭClO2��Һ��Ũ��Ϊ mol��L���ú���ĸ�Ĵ���ʽ��ʾ��������֪2 Na2S2O3+I2= Na2S4O6+2NaI��
����Na+��Ba2+��Cu2+��SO42����Cl�� ����γɵ�����ǿ�������Һ���ֱ�װ����ͼװ��
�еļס��ҡ��������ձ��н��е�⣬�缫��Ϊʯī�缫��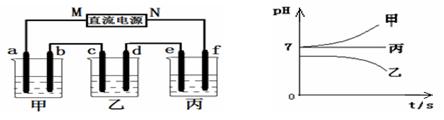
��ͨ��Դ������һ��ʱ��������c�缫�������ӡ������¸��ձ�����ҺpH����ʱ��t�Ĺ�ϵ������ͼ�������������ܽ������Ӱ�죩���ݴ˻ش��������⣺
��1��д�����ձ��з�����Ӧ�Ļ�ѧ����ʽ ��
��2���缫f�Ϸ����ĵ缫��ӦΪ ��
��3��������һ��ʱ�������ձ���c�缫����������8g��Ҫʹ���ձ�����Һ�ָ���ԭ����״̬��Ӧ���еIJ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾˮ�����Թ�����һö���������������۲죺
(1)�����������⣬�������ĸ�ʴ����________��ʴ��
(2)���Թ���Һ����������ԭ��Һ��________�ԣ�����________��ʴ���缫��ӦʽΪ��������____________________��������____________________��
(3)���Թ���Һ���½�����ԭ��Һ��________�ԣ�����________��ʴ���缫��ӦʽΪ��������____________________��������____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij����С���������ͼ��ʾ��װ�ã����ڻ������������Ƶ���ǿ�����е������������л������NaCl��Һ�����ʵ��(��ʱ����ֹˮ��a���ر�ֹˮ��b)�����ڴ��ģ�ʵ�鲢δ�ﵽԤ��Ŀ�ģ���Ҳ�����˺����˸��˵�����(�����ӽ���Ĥֻ���������Ӻ�ˮͨ��)
��������Ƿ������ش��������⣺
(1)д��Bװ���еĵ缫��Ӧ��
������________��������________��
(2)�۲쵽Aװ���е������ǣ���________����________����________��
(3)���۲쵽Aװ���е���������ǹر�ֹˮ��a����ֹˮ��b���ٹ۲�Cװ�ã�����������˵�����ɣ�����������д���йط�Ӧ�Ļ�ѧ����ʽ(�����ӷ�Ӧ��д���ӷ���ʽ)��____________________________________��
(4)����ﵽ���NaCl��Һ��Ŀ�ģ�Ӧ��θĽ�װ�ã��������������
__________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ͼ��ʾ�����γ�����ȼ�ϵ�ء�ͨ������ȼ�ϵ������ʽ(���������ҺΪ����ʱ)�ͼ�ʽ�����������ҺΪNaOH(aq)��KOH(aq)ʱ�����֡��Իش��������⣺
(1)��ʽ��صĵ缫��Ӧ������_______________������______________������ܷ�Ӧ��________________��
�������ҺpH�ı仯________(������С�����䡱)��
(2)��ʽ��صĵ缫��Ӧ������_________________������_________________������ܷ�Ӧ��______________���������ҺpH�ı仯________(������С�����䡱)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪Ǧ���صĹ���ԭ��ΪPb+PbO2+2H2SO4  2PbSO4+2H2O��������ͼװ�ý��е��(���Һ����)����õ�Ǧ������ת��0.4 mol ����ʱ���缫����������11.2 g����ش��������⡣
2PbSO4+2H2O��������ͼװ�ý��е��(���Һ����)����õ�Ǧ������ת��0.4 mol ����ʱ���缫����������11.2 g����ش��������⡣
(1)A��Ǧ���ص� ����Ǧ����������ӦʽΪ ���ŵ�����е��Һ���ܶ� (���С�����������䡱)��
(2)Ag�缫�ĵ缫��Ӧʽ�� ���õ缫�ĵ缫���ﹲ g��
(3)Cu�缫�ĵ缫��Ӧʽ�� ��CuSO4��Һ��Ũ�� (���С�����������䡱)��
(4)��ͼ��ʾ�����й�����ij����(������x)��ʱ��ı仯���ߣ���x��ʾ ��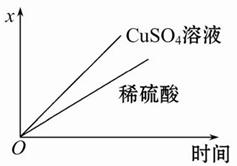
a.��U�ι��в�������������
b.��U�ι������������ļ�����
c.��U�������������������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com