
ЁОЬтФПЁПТШЦјЪЧвЛжжживЊЕФЛЏЙЄдСЯЁЃ
ЃЈ1ЃЉТШЦјКЭЪЏЛвШщЗДгІПЩвджЦЕУЦЏАзЗлЃЌЕБЦЏАзЗлБЉТЖдкПеЦјжавђЮќЪеСЫПеЦјжаЕФCO2КЭH2OЖјПЩФмВПЗжБфжЪЃЌаДГібщжЄЦЏАзЗлвбВПЗжБфжЪЕФЪЕбщЗНЗЈЃК________ЁЃ
ЃЈ2ЃЉТШЫЎжаКЌгаЖржжГЩЗжЃЌвђЖјОпгаЖржжаджЪЃЌИљОнаТжЦТШЫЎЗжБ№гыШчЭМЫФжжЮяжЪЗЂЩњЕФЗДгІЬюПеЃЌaЁЂbЁЂcЁЂdжажиКЯВПЗжДњБэЮяжЪМфЗДгІЃЌЧвТШЫЎзуСПЁЃ
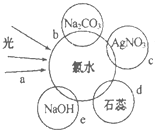
cЙ§ГЬЕФРызгЗНГЬЪНЮЊ_______ЁЃ
eЙ§ГЬжабѕЛЏЛЙдЗДгІЕФЛЏбЇЗНГЬЪНЮЊ________ЁЃ
dЙ§ГЬЫљЙлВьЕНЕФЯжЯѓЮЊ_______ЁЃ
bЙ§ГЬжЄУїСЫТШЫЎжаДцдк_________ЃЈЬюзжФИЃЉЮЂСЃЁЃ
A Cl2 B Cl- C HClO DH+
ЃЈ3ЃЉИљОнЯТБэБэШѕЫсЕФЕчРыГЃЪ§ЃЌаДГіЖўжжПЩвддіМгБЅКЭТШЫЎжаЕФДЮТШЫсЕФХЈЖШЕФФЦбЮ_______ЁЃЫЕУїбЁдёЕФРэгЩЛђЬѕМўЃК_________ЁЃ
ФГаЉШѕЫсЕФЕчРыГЃЪ§ЃЈ298KЃЉ | ||
ЖрдЊШѕЫс | K1 | K2 |
HClO | 2.95ЁС10-8 | |
CH3COOH | 1.76ЁС10-5 | |
H2SO3 | 1.54ЁС10-2 | 1.02ЁС10-17 |
H2CO3 | 4.30ЁС10-7 | 5.61ЁС10-11 |
ЁОД№АИЁПШЁбљЃЌгУВЃСЇАєеКШЁЩйСПЦЏАзЗлШмвКЕЮдкЪЏШяЪджНЩЯЃЌЪЏШяЪджНЭЪЩЋЃЛдйШЁбљЃЌМгШыбЮЫсЃЌгаЦјХнВњЩњЃЌЫЕУїЦЏАзЗлвбВПЗжБфжЪ Ag++Cl-=AgClЁ§ Cl2+2NaOH=NaCl+NaClO+H2O ЪЏШяЯШБфКьКѓЭЪЩЋ D NaHCO3ЁЂCH3COONa МгШыЕФФЦбЮжЛФмгыбЮЫсЗДгІЃЌВЛФмгыДЮТШЫсЗДгІЃЌФмЪЙЗДгІCl2+H2O![]() HCl+HClOе§ЯђвЦЖЏ
HCl+HClOе§ЯђвЦЖЏ
ЁОНтЮіЁП
ЃЈ1ЃЉЦЏАзЗлБЉТЖдкПеЦјжавђЮќЪеСЫПеЦјжаЕФCO2КЭH2OЖјПЩФмВПЗжБфжЪЃЌДЫЙ§ГЬЗЂЩњЕФЗДгІЮЊЃКCa(ClO)2+CO2+H2O=2HClO+CaCO3Ё§ЃЌвЊЯыбщжЄЦЏАзЗлвбВПЗжБфжЪЃЌжЛашбщжЄДЫБфжЪЗДгІЕФВњЮяМДПЩЁЃHClOгаЦЏАзадЃЌОнДЫШЁбљЃЌгУВЃСЇАєеКШЁЩйСПЦЏАзЗлШмвКЕЮдкЪЏШяЪджНЩЯЃЌЪЏШяЪджНЭЪЩЋЁЃCaCO3ПЩвдКЭбЮЫсЗДгІЩњГЩЖўбѕЛЏЬМЃЌОнДЫдйШЁбљЃЌМгШыбЮЫсЃЌгаЦјХнВњЩњЁЃзлЩЯЫљЪіЃЌбщжЄЦЏАзЗлвбВПЗжБфжЪЕФЪЕбщЗНЗЈЮЊЃКШЁбљЃЌгУВЃСЇАєеКШЁЩйСПЦЏАзЗлШмвКЕЮдкЪЏШяЪджНЩЯЃЌЪЏШяЪджНЭЪЩЋЃЛдйШЁбљЃЌМгШыбЮЫсЃЌгаЦјХнВњЩњЃЌЫЕУїЦЏАзЗлвбВПЗжБфжЪЁЃ
Д№АИЮЊЃКШЁбљЃЌгУВЃСЇАєеКШЁЩйСПЦЏАзЗлШмвКЕЮдкЪЏШяЪджНЩЯЃЌЪЏШяЪджНЭЪЩЋЃЛдйШЁбљЃЌМгШыбЮЫсЃЌгаЦјХнВњЩњЃЌЫЕУїЦЏАзЗлвбВПЗжБфжЪЁЃ
ЃЈ2ЃЉаТжЦТШЫЎЕФГЩЗжгаЃКCl2ЁЂHClOЁЂH2OЁЂH+ЁЂCl-ЁЂClO-ЁЂOH-(ЩйСП)ЃЌcЙ§ГЬЪЧТШЫЎжаЕФCl-КЭAg+ЗДгІЕФЙ§ГЬЃЌРызгЗНГЬЪНЮЊЃКAg++Cl-=AgClЁ§ЃЛeЙ§ГЬЪЧТШЫЎжаЕФCl2КЭNaOH
ЗДгІЃЌЛЏбЇЗНГЬЪНЮЊЃКCl2+2NaOH=NaCl+NaClO+H2OЃЛаТжЦТШЫЎжаКЌгабЮЫсКЭДЮТШЫсЃЌбЮЫсЪЧЧПЫсЃЌПЩвдЪЙзЯЩЋЪЏШяБфКьЃЌДЮТШЫсгаЦЏАзадЃЌЫљвдdЙ§ГЬЫљЙлВьЕНЕФЯжЯѓЮЊЃКЯШБфКьКѓЭЪЩЋЃЛbЙ§ГЬЪЧТШЫЎжаЕФбЮЫсКЭЬМЫсФЦЗДгІЩњГЩЖўбѕЛЏЬМЃЌжЄУїТШЫЎжагаH+ЃЌД№АИбЁDЃЛ
Д№АИЮЊЃКAg++Cl-=AgClЁ§ЃЛCl2+2NaOH=NaCl+NaClO+H2OЃЛЪЏШяЯШБфКьКѓЭЪЩЋЃЛDЃЛ
ЃЈ3ЃЉТШЫЎжаДЮТШЫсРДдДгкЃКCl2+H2O![]() HCl+HClOЃЌвЊЯыдіМгБЅКЭТШЫЎжаЕФДЮТШЫсЕФХЈЖШЃЌашЪЙИУЗДгІЦНКте§ЯђвЦЖЏЃЌПЩвдЭЈЙ§ЯћКФбЮЫсЕФЗНЗЈЪЕЯжЁЃФмдіМгБЅКЭТШЫЎжаЕФДЮТШЫсХЈЖШЕФФЦбЮЃЌгІТњзуЃККЭбЮЫсЗДгІЖјгжВЛКЭHClOЗДгІЃЌИљОнБэИёжаИјГіЕФЕчРыГЃЪ§Ъ§жЕЃЌПЩвдЕУГіЫсадЧПШѕЮЊЃКCH3COOH> H2CO3> HClO > HCO3-ЃЌЫљвдГЃМћЕФЗћКЯЬѕМўЕФФЦбЮПЩвдЪЧNaHCO3КЭCH3COONaЁЃ
HCl+HClOЃЌвЊЯыдіМгБЅКЭТШЫЎжаЕФДЮТШЫсЕФХЈЖШЃЌашЪЙИУЗДгІЦНКте§ЯђвЦЖЏЃЌПЩвдЭЈЙ§ЯћКФбЮЫсЕФЗНЗЈЪЕЯжЁЃФмдіМгБЅКЭТШЫЎжаЕФДЮТШЫсХЈЖШЕФФЦбЮЃЌгІТњзуЃККЭбЮЫсЗДгІЖјгжВЛКЭHClOЗДгІЃЌИљОнБэИёжаИјГіЕФЕчРыГЃЪ§Ъ§жЕЃЌПЩвдЕУГіЫсадЧПШѕЮЊЃКCH3COOH> H2CO3> HClO > HCO3-ЃЌЫљвдГЃМћЕФЗћКЯЬѕМўЕФФЦбЮПЩвдЪЧNaHCO3КЭCH3COONaЁЃ
Д№АИЮЊЃКNaHCO3ЁЂCH3COONaЃЛМгШыЕФФЦбЮжЛФмгыбЮЫсЗДгІЃЌВЛФмгыДЮТШЫсЗДгІЃЌФмЪЙЗДгІCl2+H2O![]() HCl+HClOе§ЯђвЦЖЏЃЛ
HCl+HClOе§ЯђвЦЖЏЃЛ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвЖВѕЩЂЃЈisoprocardЃЉЖдЫЎЕОвЖВѕКЭЗЩЪОпгаНЯЧПЕФДЅЩБзїгУЃЌЗРаЇбИЫйЃЌЕЋВааЇВЛГЄЃЎЙЄвЕЩЯгУСквьБћЛљБНЗгКЯГЩвЖВѕЩЂЕФЙ§ГЬШчЯТЃК

ЯТСагаЙиЫЕЗЈе§ШЗЕФЪЧЃЈЁЁЁЁЃЉ
A.вЖВѕЩЂЕФЗжзгЪНЪЧC11H16NO2
B.СквьБћЛљБНЗгЗЂЩњСЫШЁДњЗДгІ
C.вЖВѕЩЂдкЧПЫсЁЂЧПМюадЛЗОГжаФмЮШЖЈДцдк
D.ПЩгУFeCl3МьбщвЖВѕЩЂжаЪЧЗёКЌСквьБћЛљБНЗг
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЭАхЩЯЬњУЖЄДІЕФЮќбѕИЏЪДдРэШчЭМЫљЪОЃЌЯТСагаЙиЫЕЗЈжаЃЌВЛе§ШЗЕФЪЧ

A. е§МЋЕчМЋЗДгІЪНЮЊЃК2H++2eЁЊЁњH2Ёќ
B. ДЫЙ§ГЬжаЛЙЩцМАЕНЗДгІЃК4Fe(OH)2+2H2O+O2=4Fe(OH)3
C. ДЫЙ§ГЬжаЭВЂВЛБЛИЏЪД
D. ДЫЙ§ГЬжаЕчзгДгFeвЦЯђCu
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПБНМзЫсЃЈ![]() ЃЉЪЧживЊЕФЛЏЙЄдСЯЃЌПЩгІгУгкЯћЖОЗРИЏЁЂШОСЯдиЬхЁЂдіЫмМСЁЂЯуСЯМАЪГЦЗЗРИЏМСЕФЩњВњЃЌвВПЩгУгкИжЬњЩшБИЕФЗРатМСЁЃФГЛЏбЇЪЕбщаЁзщдкЪЕбщЪвжавдБНМзШЉЮЊдСЯжЦШЁБНМзЫсКЭИБВњЦЗБНМзДМЃЈ
ЃЉЪЧживЊЕФЛЏЙЄдСЯЃЌПЩгІгУгкЯћЖОЗРИЏЁЂШОСЯдиЬхЁЂдіЫмМСЁЂЯуСЯМАЪГЦЗЗРИЏМСЕФЩњВњЃЌвВПЩгУгкИжЬњЩшБИЕФЗРатМСЁЃФГЛЏбЇЪЕбщаЁзщдкЪЕбщЪвжавдБНМзШЉЮЊдСЯжЦШЁБНМзЫсКЭИБВњЦЗБНМзДМЃЈ![]() ЃЉЕФЪЕбщСїГЬ:
ЃЉЕФЪЕбщСїГЬ:
вбжЊЃКЂй ЃЛ
ЃЛ![]() ЃЛ
ЃЛ ЃЈRЁЂR1БэЪОЬўЛљЛђЧтдзгЃЉ
ЃЈRЁЂR1БэЪОЬўЛљЛђЧтдзгЃЉ
ЂкЯрЙиЮяжЪЕФВПЗжЮяРэаджЪМћБэЃК
УћГЦ | ЯрЖдУмЖШ | ШлЕу/Ёц | ЗаЕу/Ёц | ШмНтЖШ | |
ЫЎ | ввУб | ||||
БНМзШЉ | 1.04 | Ѓ26 | 179.6 | ЮЂШм | взШм |
БНМзЫс | 1.27 | 122.1 | 249 | 25ЁцЮЂШмЃЌ95ЁцПЩШм | взШм |
БНМзДМ | 1.04 | Ѓ15.3 | 205.7 | ЮЂШм | взШм |
ввУб | 0.71 | Ѓ116.3 | 34.6 | ВЛШм | ЁЊ |
ЧыЛиД№ЯТСаЮЪЬт:
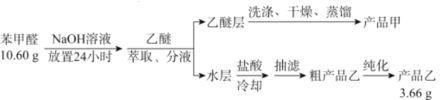
ЃЈ1ЃЉНјаанЭШЁЁЂЗжвКВйзїЪБЫљгУВЃСЇвЧЦїЕФУћГЦЮЊ___________ЁЃЗжвКЪБЃЌввУбВугІДг_______(ЬюЁАЯТПкЗХГіЁБЛђЁАЩЯПкЕЙГіЁБ)ЁЃ
ЃЈ2ЃЉЯДЕгввУбВуЪБашвЊвРДЮгУNaHSO3ШмвКЁЂ10%Na2CO3ШмвКЁЂеєСѓЫЎНјааЯДЕгЁЃЦфжаМгШыNaHSO3ШмвКЯДЕгЕФжївЊФПЕФЪЧ________________ЃЌЖдгІЕФЛЏбЇЗНГЬЪНЮЊ___________________________ЁЃ
ЃЈ3ЃЉеєСѓЛёЕУВњЦЗМзЪБМгШыЫщДЩЦЌЕФФПЕФЮЊ_____________ЃЌеєСѓЪБгІПижЦЮТЖШдк____ЁцзѓгвЁЃ
A.34.6 B.179.6 C.205.7 D.249
ЃЈ4ЃЉЬсДПДжВњЦЗввЛёЕУВњЦЗввЕФДПЛЏЗНЗЈУћГЦЮЊ________________ЁЃ
ЃЈ5ЃЉГЦШЁ10.60gЕФБНМзШЉНјааЪЕбщЃЌзюжежЦШЁВњЦЗввЕФжЪСПЮЊ3.66gЃЌдђВњЦЗввЕФВњТЪЮЊ____________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПдквЛЖЈЮТЖШЯТЕФЖЈШнУмБеШнЦїжаЃЌЕБЯТСаЮяРэСПВЛдйИФБфЪБЃЌВЛФмБэУїЗДгІA(s)ЃЋ2B(g)![]() C(g)ЃЋD(g)вбДяЦНКтЕФЪЧ
C(g)ЃЋD(g)вбДяЦНКтЕФЪЧ
A.ЛьКЯЦјЬхЕФбЙЧПB.ЛьКЯЦјЬхЕФУмЖШ
C.ЛьКЯЦјЬхЕФЯрЖдЗжзгжЪСПD.CЕФЮяжЪЕФСП
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁППрагШЪЫсдквНвЉЙЄвЕПЩгУгкКЯГЩЭЗцпєЧпђЁЂєЧмапђЁЂЦЅФЊСжЕШЕФжаМфЬхЃЌЯТСаТЗЯпЪЧКЯГЩПрагШЪЫсМАЦфбмЩњЮяЕФвЛжжЗНЗЈЃК
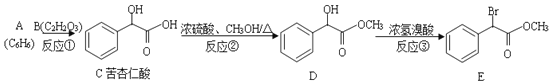
ЃЈ1ЃЉПрагШЪЫсжаКЌгаЕФЙйФмЭХУћГЦ__________ЁЃ
ЃЈ2ЃЉЩњГЩCЕФЗДгІРраЭ_________ЁЃBЕФНсЙЙМђЪН____________ЁЃ
ЃЈ3ЃЉ1molEзюЖрПЩвдгы___________NaOHЗДгІЁЃ
ЃЈ4ЃЉЗДгІЂлЕФЛЏбЇЗНГЬЪНЮЊ_______ЁЃ
ЃЈ5ЃЉ СНИіCЗжзгПЩвдЗДгІЩњГЩОпгаШ§ИіСљдЊЛЗЕФЛЏКЯЮяFЃЌдђFЕФНсЙЙМђЪНЮЊ______ЁЃ
ЃЈ6ЃЉаДГіТњзуЯТСаЬѕМўЕФCЕФЭЌЗжвьЙЙЬхЃЌМШФмЗЂЩњвјОЕЗДгІЃЌгжФмЗЂЩњЫЎНтЗДгІЃЌгіЕНFeCl3ШмвКЯдзЯЩЋЃЌЦфжаБНЛЗЩЯжЛгаСНИіЮЛгкЖдЮЛШЁДњЛљЃЌЦфНсЙЙМђЪНЮЊ________ЁЃ
ЃЈ7ЃЉвбжЊЃКRCH2COOH![]() RCHClCOOHЃЌЧывдБљДзЫсЮЊдСЯЃЈЮоЛњЪдМСШЮбЁЃЉЩшМЦжЦБИОлввДМЫсЃЈ
RCHClCOOHЃЌЧывдБљДзЫсЮЊдСЯЃЈЮоЛњЪдМСШЮбЁЃЉЩшМЦжЦБИОлввДМЫсЃЈ![]() ЃЉЕФКЯГЩТЗЯп______ЁЃ
ЃЉЕФКЯГЩТЗЯп______ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПМзДМзїЮЊШМСЯЃЌдкЛЏЪЏФмдДКЭПЩдйЩњФмдДЪБЦкОљгаЙуЗКЕФгІгУЧАОАЁЃ
I. МзДМПЩвдЬцДњЦћгЭКЭВёгЭзїЮЊФкШМЛњШМСЯЁЃ
ЃЈ1ЃЉЦћгЭЕФжївЊГЩЗжжЎвЛЪЧаСЭщ[C8H18(l)]ЁЃвбжЊЃК25ЁцЁЂ101 kPaЪБЃЌ1 mol C8H18(l)ЭъШЋШМЩеЩњГЩЦјЬЌЖўбѕЛЏЬМКЭвКЬЌЫЎЃЌЗХГі5518 kJШШСПЁЃИУЗДгІЕФШШЛЏбЇЗНГЬЪНЮЊ______ЁЃ
ЃЈ2ЃЉвбжЊЃК25ЁцЁЂ101 kPaЪБЃЌCH3OH(l) + 3/2 O2(g) ==== CO2 (g) + 2H2O(l) ІЄ HЃН-726.5 kJ/molЁЃЯрЭЌжЪСПЕФМзДМКЭаСЭщЗжБ№ЭъШЋШМЩеЪБЃЌЗХГіШШСПНЯЖрЕФЪЧ______ЁЃ
ЃЈ3ЃЉФГбаОПепЗжБ№вдМзДМКЭЦћгЭзіШМСЯЃЌЪЕбщВтЕУдкЗЂЖЏЛњИпИККЩЙЄзїЧщПіЯТЃЌЦћГЕЮВЦјжаCOЕФАйЗжКЌСПгыЦћГЕЕФМгЫйадФмЕФЙиЯЕШчгвЫљЪОЁЃ
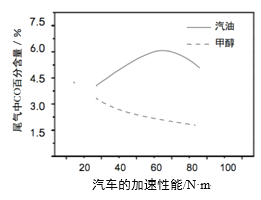
ИљОнЭМаХЯЂЗжЮіЃЌгыЦћгЭЯрБШЃЌМзДМзїЮЊШМСЯЕФгХЕуЪЧ______ЁЃ
II. МзДМЕФКЯГЩ
ЃЈ4ЃЉвдCO2(g)КЭH2(g)ЮЊдСЯКЯГЩМзДМЃЌЗДгІЕФФмСПБфЛЏШчЯТЭМЫљЪОЁЃ
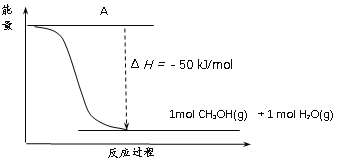
Ђй ВЙШЋЩЯЭМЃКЭМжаAДІгІЬюШы______ЁЃ
Ђк ИУЗДгІашвЊМгШыЭЃаПЛљДпЛЏМСЁЃМгШыДпЛЏМСКѓЃЌИУЗДгІЕФІЄH______ЃЈЬюЁАБфДѓЁБЁАБфаЁЁБЛђЁАВЛБфЁБЃЉЁЃ
ЃЈ5ЃЉвбжЊЃК CO(g)ЃЋ1/2 O2(g) ==== CO2(g) ІЄH1ЃН-283 kJ/mol
H2(g)ЃЋ1/2 O2(g) ==== H2O(g) ІЄH2ЃН-242 kJ/mol
CH3OH(g) + 3/2 O2(g) ==== CO2 (g) + 2H2O(g) ІЄH3ЃН-676 kJ/mol
вдCO(g)КЭH2(g)ЮЊдСЯКЯГЩМзДМЕФЗДгІЮЊCO(g) + 2H2(g) ==== CH3OH(g) ЁЃИУЗДгІЕФІЄHЮЊ_____ kJ/molЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЫЕЗЈВЛе§ШЗЕФЪЧ(ЁЁЁЁ)
A. HClЁЂHBrЁЂHIЕФШлЁЂЗаЕувРДЮЩ§ИпгыЗжзгМфзїгУСІДѓаЁгаЙи
B. H2OЕФШлЁЂЗаЕуИпгкH2SЪЧгЩгкH2OЗжзгМфДцдкЧтМќ
C. МзЭщПЩгыЫЎаЮГЩЧтМќ
D. АзОЦжаЃЌввДМЗжзгКЭЫЎЗжзгМфДцдкЗЖЕТЛЊСІКЭЧтМќ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПМюЪНбѕЛЏФј(NiOOH)ПЩгУзїФјЧтЕчГиЕФе§МЋВФСЯЃЌПЩгУЗЯФјДпЛЏМСЃЈжївЊКЌNiЁЂAlЃЌЩйСПCrЁЂFeS ЕШ)РДжЦБИЃЌЦфЙЄвеСїГЬШчЯТЃК

ЛиД№ЯТСаЮЪЬтЃК
(1)ЁАНўХнГ§ТСЁБЪБЃЌЗЂЩњЗДгІЕФРызгЗДгІЗНГЬЪНЮЊ_________________________;
(2)ЁАШмНтЁБЪБЗХГіЕФЦјЬхЮЊ_______________ (ЬюЛЏбЇЪН);
(3)вбжЊИУЬѕМўЯТН№ЪєРызгПЊЪМГСЕэКЭЭъШЋГСЕэЕФpHШчЯТБэЃК
ПЊЪМГСЕэЕФpH | ЭъШЋГСЕэЕФpH | |
Ni2+ | 6.2 | 8.6 |
Fe2+ | 7.6 | 9.1 |
Fe3+ | 2.3 | 3.3 |
Cr3+ | 4.5 | 5.6 |
ЁАЕїpH 1ЁБЪБЃЌШмвКpHЗЖЮЇЮЊ______________________ЃЛ
(4)дкПеЦјжаМгШШNi(OH)2ПЩЕУNiOOH,ЧыаДГіДЫЗДгІЕФЛЏбЇЗНГЬЪН_____________;

(5)Н№ЪєИѕдкШмвКжагаЖржжДцдкаЮЪН, CrO42ЁЊКЭCr2O72ЁЊдкШмвКжаПЩЯрЛЅзЊЛЏЁЃЪвЮТЯТЃЌГѕЪМХЈЖШЮЊ1.0mol/LЕФNa2CrO4ШмвКжаc(Cr2O72ЁЊ)Ыцc(H+)ЕФБфЛЏШчЭМЫљЪОЃЌгУРызгЗНГЬЪНБэЪОNa2CrO4ШмвКжаЕФзЊЛЏЗДгІ________________ЃЌИљОнAЕуЪ§ОнМЦЫуГіИУзЊЛЏЗДгІЕФЦНКтГЃЪ§ЮЊ______________,ЮТЖШЩ§ИпЃЌШмвКжаCrO42ЁЊЕФЦНКтзЊЛЏТЪМѕаЁЃЌдђИУЗДгІЕФЁїH____0ЃЈЬюЁА>ЁБЁЂЁА<ЁБЛђЁА=ЁБЃЉЁЃ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com