
ЁОЬтФПЁПгаЛњЮяI(ЗжзгЪНЮЊC19H20O4)ЪєгкЗМЯуѕЅРрЮяжЪЃЌЪЧвЛжжЕїЯуМСЃЌЦфКЯГЩТЗЯпШчЯТЃК

вбжЊЃКЂйAЪєгкжЌЗОЬўЃЌКЫДХЙВеёЧтЦзЯдЪОга2зщЗхЃЌУцЛ§БШЮЊ3ЃК1ЃЌЦфеєЦјУмЖШЪЧЯрЭЌЬѕМўЯТH2ЕФ28БЖЃЛDЗжзгЪНЮЊC4H8O3ЃЛEЗжзгЪНЮЊC4H6O2ЃЌФмЪЙфхЫЎЭЪЩЋЁЃ
Ђк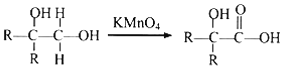 ЦфжаRЮЊЬўЛљЁЃ
ЦфжаRЮЊЬўЛљЁЃ
Ђл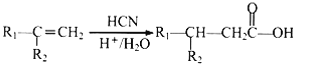 ЦфжаR1КЭR2ОљЮЊЬўЛљЁЃ
ЦфжаR1КЭR2ОљЮЊЬўЛљЁЃ
ЛиД№ЯТСаЮЪЬтЃК
(1)AЕФНсЙЙМђЪНЮЊ___________________ЁЃ
(2)DжаЙйФмЭХЕФУћГЦЪЧ______________________ЁЃ
(3)ЗДгІЂкЕФЛЏбЇЗНГЬЪНЮЊ______________ЁЃ
(4)EЕФЭЌЯЕЮяKБШEЖрвЛИіЬМдзгЃЌKгаЖржжСДзДЭЌЗжвьЙЙЬхЃЌЦфжаФмЗЂЩњвјОЕЗДгІЧвФмЫЎНтЕФга_______жжЁЃ
(5)ЗДгІЂйЁЋЂпЪєгкШЁДњЗДгІЕФЪЧ__________(ЬюађКХ)ЁЃ
(6)ЗДгІЂпЕФЛЏбЇЗНГЬЪНЮЊ_________________ЁЃ
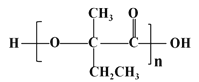
(7)ВЮееЩЯЪіКЯГЩТЗЯпЃЌвд 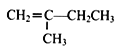 ЮЊдСЯ(ЮоЛњЪдМСШЮбЁ)ЃЌО4ВНЗДгІжЦБИПЩНЕНтЫмСЯ
ЮЊдСЯ(ЮоЛњЪдМСШЮбЁ)ЃЌО4ВНЗДгІжЦБИПЩНЕНтЫмСЯ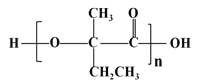 _____________ЁЃ
_____________ЁЃ
ЁОД№АИЁПCH3C(CH3)=CH2 єЧЛљЁЂєШЛљ CH3CBr(CH3)=CH2Br+2NaOH![]() CH3C(OH)(CH3)CH2OH+2NaBr 8 ЂкЂнЂоЂп
CH3C(OH)(CH3)CH2OH+2NaBr 8 ЂкЂнЂоЂп 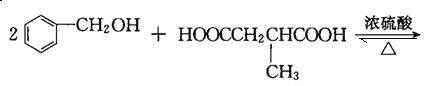
![]()
ЁОНтЮіЁП
ИљОнвбжЊЂйM(A)=28ЁС2=56gЁЄmo1-1,AЪєгкжЌЗОЬў,AЕФЗжзгЪНЮЊC4H8,AЕФКЫДХЙВеёЧтЦзЯдЪОга2зщЗхЧвУцЛ§БШЮЊ3:1,AЕФНсЙЙМђЪНЮЊCH3C(CH3)=CH2ЃЛИљОнИїВНЗДгІЬѕМў![]() ПЩжЊЃКBЕФНсЙЙМђЪНЮЊCH3CBr(CH3)=CH2Br,CЕФНсЙЙМђЪНЮЊCH3C(OH)(CH3)CH2OH,НсКЯ
ПЩжЊЃКBЕФНсЙЙМђЪНЮЊCH3CBr(CH3)=CH2Br,CЕФНсЙЙМђЪНЮЊCH3C(OH)(CH3)CH2OH,НсКЯ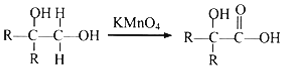 ПЩжЊDЮЊCH3C(OH)(CH3)COOH,гЩEЗжзгЪНЮЊC4H6O2ЃЌФмЪЙфхЫЎЭЪЩЋЃЌПЩжЊEЮЊCH2=C(CH3)COOHЃЌИљОнаХЯЂ
ПЩжЊDЮЊCH3C(OH)(CH3)COOH,гЩEЗжзгЪНЮЊC4H6O2ЃЌФмЪЙфхЫЎЭЪЩЋЃЌПЩжЊEЮЊCH2=C(CH3)COOHЃЌИљОнаХЯЂ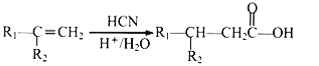 ПЩжЊFЮЊHOOCCH2CH(CH3)COOHЁЃгЩПђЭМ
ПЩжЊFЮЊHOOCCH2CH(CH3)COOHЁЃгЩПђЭМ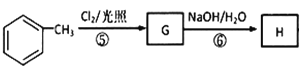 ИљОнЗДгІЬѕМўе§ЭЦПЩжЊGЮЊ
ИљОнЗДгІЬѕМўе§ЭЦПЩжЊGЮЊ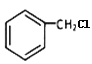 ЃЌHЮЊ
ЃЌHЮЊ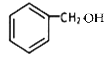 ЁЃ
ЁЃ
(1)ИљОнвбжЊЂйM(A)=28ЁС2=56gЁЄmo1-1,AЪєгкжЌЗОЬў,гУЁАЩЬЗЈЁБ,56ЁТ12=4гр8,AЕФЗжзгЪНЮЊC4H8,AЕФКЫДХЙВеёЧтЦзЯдЪОга2зщЗхЧвУцЛ§БШЮЊ3:1,дђAЕФНсЙЙМђЪНЮЊCH3C(CH3)=CH2;Д№АИЃКCH3C(CH3)=CH2ЁЃ
(2)ИљОнЩЯЪіЗжЮіDЮЊCH3C(OH)(CH3)COOHЃЌЦфжаКЌгаЙйФмЭХЕФУћГЦЪЧєЧЛљЁЂєШЛљЃЛД№АИЃКєЧЛљЁЂєШЛљЁЃ
(3)ЗДгІЂк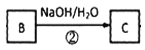 ЃЌгЩBЮЊCH3CBr(CH3)=CH2Br,CЮЊCH3C(OH)(CH3)CH2OHЃЌЗЂЩњЫЎНтЗДгІЃЌЗДгІЕФЛЏбЇЗНГЬЪНЮЊCH3CBr(CH3)=CH2Br+2NaOH
ЃЌгЩBЮЊCH3CBr(CH3)=CH2Br,CЮЊCH3C(OH)(CH3)CH2OHЃЌЗЂЩњЫЎНтЗДгІЃЌЗДгІЕФЛЏбЇЗНГЬЪНЮЊCH3CBr(CH3)=CH2Br+2NaOH![]() CH3C(OH)(CH3)CH2OH+2NaBrЁЃД№АИЃКCH3CBr(CH3)=CH2Br+2NaOH
CH3C(OH)(CH3)CH2OH+2NaBrЁЃД№АИЃКCH3CBr(CH3)=CH2Br+2NaOH![]() CH3C(OH)(CH3)CH2OH+2NaBrЁЃ
CH3C(OH)(CH3)CH2OH+2NaBrЁЃ
(4)гЩEЮЊCH2=C(CH3)COOHЃЌEЕФЗжзгЪНЮЊC4H6O2,EЕФЭЌЯЕЮяKБШEЖр-ИіЬМдзг,KЕФЗжзгЪНЮЊC5H8O2ЃЌKЕФВЛБЅКЭЖШЮЊ2,KЕФЭЌЗжвьЙЙЬхФмЗЂЩњвјОЕЗДгІЧвФмЫЎНтЃЌНсКЯЫљКЌOдзгИіЪ§,KЕФЭЌЗжвьЙЙЬхЮЊМзЫсФГѕЅЃЌЗћКЯЬѕМўЕФСДзДЭЌЗжвьЙЙЬхгаHOOOCH=CHCH2CH3(вЦЖЏЫЋМќга3жж)ЁЂHCOOC(CH3)=CHCH3(вЦЖЏЫЋМќКЭ-CH3га5жж)ЙВга8жжЃЛД№АИЃК8ЁЃ
(5)ЗДгІЂй![]() ЂпЕФЗДгІРраЭвРДЮЮЊМгГЩЗДгІЁЂШЁДњЗДгІ(ЛђЫЎНтЗДгІ)ЁЂбѕЛЏЗДгІЁЂЯћШЅЗДгІ,ШЁДњЗДгІЁЂШЁДњЗДгІ(ЛђЫЎНтЗДгІ)ЁЂШЁДњЗДгІЛђѕЅЛЏЗДгІ),ЪєгкШЁДњЗДгІЕФЪЧЂкЂнЂоЂпЁЃД№АИЃКЂкЂнЂоЂпЁЃ
ЂпЕФЗДгІРраЭвРДЮЮЊМгГЩЗДгІЁЂШЁДњЗДгІ(ЛђЫЎНтЗДгІ)ЁЂбѕЛЏЗДгІЁЂЯћШЅЗДгІ,ШЁДњЗДгІЁЂШЁДњЗДгІ(ЛђЫЎНтЗДгІ)ЁЂШЁДњЗДгІЛђѕЅЛЏЗДгІ),ЪєгкШЁДњЗДгІЕФЪЧЂкЂнЂоЂпЁЃД№АИЃКЂкЂнЂоЂпЁЃ
(6)гЩПђЭМ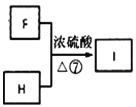 ЃЌИљОнЩЯЪіЗжЮіПЩжЊFЮЊHOOCCH2CH(CH3)COOHЃЌHЮЊ
ЃЌИљОнЩЯЪіЗжЮіПЩжЊFЮЊHOOCCH2CH(CH3)COOHЃЌHЮЊ ЃЌIЗжзгЪНЮЊC19H20O4ЃЌЗДгІЂпЕФЛЏбЇЗНГЬЪНЮЊHOOCCH2CH(CH3)COOH+2
ЃЌIЗжзгЪНЮЊC19H20O4ЃЌЗДгІЂпЕФЛЏбЇЗНГЬЪНЮЊHOOCCH2CH(CH3)COOH+2
![]()
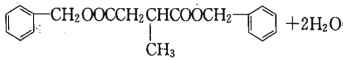 ЃЛД№АИЃКHOOCCH2CH(CH3)COOH+2
ЃЛД№АИЃКHOOCCH2CH(CH3)COOH+2
![]()
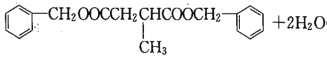 ЁЃ
ЁЃ
(7)вд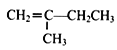 ЮЊдСЯЃЌО4ВНЗДгІжЦБИПЩНЕНтЫмСЯ
ЮЊдСЯЃЌО4ВНЗДгІжЦБИПЩНЕНтЫмСЯ ЕФКЯГЩТЗЯпЮЊЃК
ЕФКЯГЩТЗЯпЮЊЃК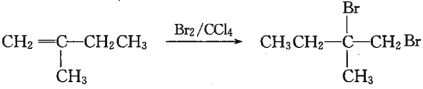
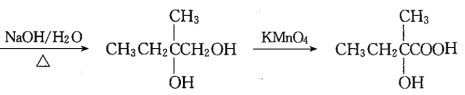
![]()
 ЁЃ
ЁЃ


 УЯНЈЦНаЁбЇЙіЖЏВтЪдЯЕСаД№АИ
УЯНЈЦНаЁбЇЙіЖЏВтЪдЯЕСаД№АИ ЛЦИдЬьЬьСЗПкЫуЬтПЈЯЕСаД№АИ
ЛЦИдЬьЬьСЗПкЫуЬтПЈЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПдквЛЖЈЬѕМўЯТN2 + 3H2 2NH3 ЕФЗДгІжаЃЌЦ№ЪМN2ХЈЖШЮЊ2mol/LЃЌH2ХЈЖШЮЊ5mol/LЃЌ ЗДгІЕН2ЗжжгЪБЃЌВтЕУ NH3 ЕФЗДгІЫйТЪЮЊv(NH3 )=0.4mol/(LЁЄmin)ЃЌЬюПеЃК
(1) NH3 ЕФзЊЛЏХЈЖШЮЊc(NH3 )=___________ mol/(LЁЄmin)
(2)Ш§ЖЮЪНЬюПеЃК
(mol/L) N2 + 3H2 2NH3
Ц№ЪМХЈЖШ 2 5 0
зЊЛЏХЈЖШ _ _ _
2minКѓХЈЖШ _ _ _
(3)гУv(N2)БэЪОЕФЗДгІЫйТЪЮЊЃКv(N2)=__________mol/(LЁЄmin)
(4)гУv(H2)БэЪОЕФЗДгІЫйТЪЮЊЃКv(H2)=__________mol/(LЁЄmin)
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
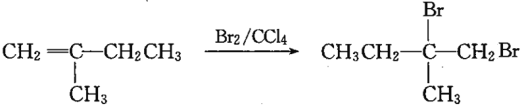
ЁОЬтФПЁПгЩCH3CH2CH2BrжЦБИCH3CH(OH)CH2OHЃЌвРДЮЗЂЩњЕФЗДгІРраЭКЭЗДгІЬѕМўЖМе§ШЗЕФЪЧ
бЁЯю | ЗДгІРраЭ | ЗДгІЬѕМў |
A | МгГЩЁЂШЁДњЁЂЯћШЅ | KOHДМШмвК/МгШШЁЂKOHЫЎШмвК/МгШШЁЂГЃЮТ |
B | ЯћШЅЁЂМгГЩЁЂШЁДњ | NaOHДМШмвК/МгШШЁЂГЃЮТЁЂKOHЫЎШмвК/МгШШ |
C | бѕЛЏЁЂШЁДњЁЂЯћШЅ | МгШШЁЂKOHДМШмвК/МгШШЁЂKOHЫЎШмвК/МгШШ |
D | ЯћШЅЁЂМгГЩЁЂЫЎНт | NaOHЫЎШмвК/МгШШЁЂГЃЮТЁЂNaOHДМШмвК/МгШШ |
A. A B. B C. C D. D
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЛ№ЩНБЌЗЂЪБЛсХчГіSO2ЃЌSO2ЪЧДѓЦјжївЊЮлШОЮяжЎвЛЃЌдкЙЄвЕжаПЩгУгкжЦБИСђЫсЁЃЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉSO2ЪЧ_____(ЬюЁАЕчНтжЪЁБЛђЁАЗЧЕчНтжЪЁБ)ЁЃ
ЃЈ2ЃЉCu2SгыO2ЗДгІПЩЩњГЩSO2ЃЌвбжЊЃК
Cu(s)+![]() O2(g)=CuO(s) ЁїH=x kJmol-1
O2(g)=CuO(s) ЁїH=x kJmol-1
Cu(s)+![]() S(s)=
S(s)=![]() Cu2S(s) ЁїH=y kJmol-1
Cu2S(s) ЁїH=y kJmol-1
S(s)+O2(g)=SO2(g) ЁїH=z kJmol-1
аДГіCu2SгыO2ЗДгІЩњГЩCuOКЭSO2ЕФШШЛЏбЇЗНГЬЪН__________________________ЁЃ
ЃЈ3ЃЉСђЫсЙЄвЕжаЩцМАЗДгІЃК2SO2(g)+O2(g)2SO3(g)ЁїH=Q kJЁЄmol-1ЁЃвЛЖЈЬѕМўЯТЃЌдк2LКуШнУмБеШнЦїжаЃЌЭЈШы2molSO2КЭ1molO2ЗЂЩњЩЯЪіЗДгІЃЌSO2ЕФЦНКтзЊЛЏТЪгыбЙЧПЁЂЮТЖШЕФЙиЯЕШчЭМЫљЪОЁЃaЕуЪБДЫЗДгІЕФЦНКтГЃЪ§ЕФЪ§жЕЮЊ_____ЁЃ
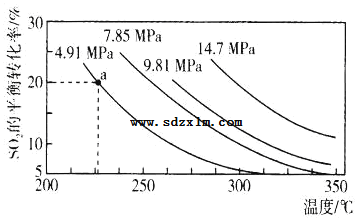
ЙигкИУЗДгІЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧ____ЁЃ
AЃЎШнЦїФкЛьКЯЦјЬхЕФУмЖШВЛдйБфЛЏЪБЃЌЗДгІДяЕНЦНКт
BЃЎЯрЭЌЪБМфФкЩњГЩ2molSO2ЭЌЪБЯћКФ1molO2ЃЌЗДгІДяЕНЦНКт
CЃЎQДѓгк0
DЃЎЯрЭЌЮТЖШЯТЃЌбЙЧПдНДѓЃЌSO2ЕФЦНКтзЊЛЏТЪОЭдНДѓЃЌИУЗДгІЕФЦНКтГЃЪ§ОЭдНДѓ
EЃЎЗДгІДяЕНЦНКтКѓБЃГжЮТЖШВЛБфЃЌдйГфШы2molSO2КЭ1molO2ЃЌSO2ЕФЦНКтзЊЛЏТЪдіДѓ
FЃЎЗДгІДяЕНЦНКтКѓБЃГжЮТЖШВЛБфЃЌдйГфШыHe(g)ЃЌSO2ЕФЦНКтзЊЛЏТЪдіДѓ
ЃЈ4ЃЉНЋSO2ЭЈШыЫсЛЏЕФЯѕЫсБЕШмвКПЩЩњГЩСђЫсБЕГСЕэЃЌ25ЁцЪБЃЌKSP(BaSO4)=1ЁС10-10ЁЃKSP(BaCO3)=2.6ЁС10-9ЁЃИУЮТЖШЯТЃЌBaSO4КЭBaCO3ГСЕэЙВДцЕФаќзЧвКжаЃЌ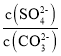 =___ЁЃ
=___ЁЃ
ЃЈ5ЃЉгУШчЭМзАжУЛиЪеSO2ПЩжЦЕУСђЫсЃЌЕчМЋЮЊЖшадЕчМЋЃЌaЁЂbФЄЗжБ№ЮЊбєРызгНЛЛЛФЄЁЂвѕРызгНЛЛЛФЄЁЃбєМЋЕФЕчМЋЗДгІЮЊ____ЁЃ
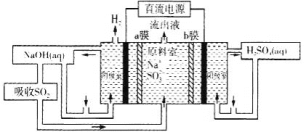
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁП1942 ФъЃЌЮвЙњЛЏЙЄзЈМвКюЕТАёвд NaClЁЂNH3ЁЂCO2 ЕШЮЊдСЯЯШжЦЕУ NaHCO3ЃЌНјЖјЩњВњГіДПМюЃЌ ЫћЕФЁАКюЪЯжЦМюЗЈЁБЮЊЪРНчжЦМюЙЄвЕзіГіСЫЭЛГіЙБЯзЁЃгаЙиЗДгІЕФЛЏбЇЗНГЬЪНШчЯТЃК
NH3ЃЋCO2ЃЋH2O=NH4HCO3 ЃЛ
NH4HCO3ЃЋNaCl=NaHCO3Ё§ЃЋNH4Cl ЃЛ
2NaHCO3![]() Na2CO3ЃЋCO2ЁќЃЋH2O
Na2CO3ЃЋCO2ЁќЃЋH2O
(1)ЁАКюЪЯжЦМюЗЈЁБАбКЯГЩАБКЭДПМюСНжжВњЦЗСЊКЯЩњВњЃЌЧыаДГіЙЄвЕКЯГЩАБЕФЛЏбЇЗДгІЗНГЬЪН_______
(2)ЬМЫсЧтяЇгыБЅКЭЪГбЮЫЎЗДгІЃЌФмЮіГіЬМЫсЧтФЦОЇЬхЕФдвђЪЧ_______ЁЃ
aЃЎЬМЫсЧтФЦФбШмгкЫЎ
bЃЎЬМЫсЧтФЦЪмШШвзЗжНт
cЃЎЬМЫсЧтФЦЕФШмНтЖШЯрЖдНЯаЁЃЌЫљвддкШмвКжаЪзЯШНсОЇЮіГі
(3)ФГЬНОПЛюЖЏаЁзщИљОнЩЯЪіжЦМюдРэЃЌгћжЦБИЬМЫсЧтФЦЃЌЭЌбЇУЧАДИїздЩшМЦЕФЗНАИНјааЪЕбщЁЃ
ЕквЛЮЛЭЌбЇЃКНЋЖўбѕЛЏЬМЦјЬхЭЈШыКЌАБЕФБЅКЭЪГбЮЫЎжажЦБИЬМЫсЧтФЦЃЌЪЕбщзАжУШчЭМЫљЪО(ЭМжаМаГжЁЂЙЬЖЈгУЕФвЧЦїЮДЛГі)ЁЃ
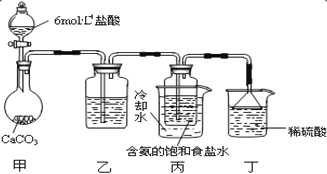
ЧыЛиД№ЃК
ЂйаДГіМзжаЗЂЩњЗДгІЕФРызгЗНГЬЪН_______ЁЃ
ЂкввзАжУжаЕФЪдМСЪЧ_______ЁЃ
ЂлЪЕбщНсЪјКѓЃЌЗжРыГі NaHCO3 ОЇЬхЕФВйзїЪЧ_______ (ЬюЗжРыВйзїЕФУћГЦ)ЁЃ
ЕкЖўЮЛЭЌбЇЃКгУШчЭМзАжУНјааЪЕбщ(ЦфЫќзАжУЮДЛГі)ЁЃ
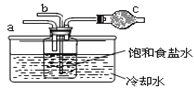
ЂйЮЊЬсИпЖўбѕЛЏЬМдкДЫЗДгІШмвКжаБЛЮќЪеЕФГЬЖШЃЌЪЕбщЪБЃЌаыЯШДг a ЙмЭЈШы_______ЦјЬхЃЌдйДгb ЙмжаЭЈШы_______ЦјЬхЁЃ
ЂкзАжУ c жаЕФЪдМСЮЊ_______(бЁЬюзжФИ)ЁЃ
eЃЎМюЪЏЛв f.ЃЎХЈСђЫс gЃЎЮоЫЎТШЛЏИЦ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁП(1)АДЯЕЭГУќУћЗЈУќУћгаЛњЮя![]() ЃК___________________ЁЃ
ЃК___________________ЁЃ
(2)аДГігаЛњЮя2,3-ЖўМзЛљ-4-ввЛљМКЭщЕФНсЙЙМђЪНЃК_______________________ЁЃ
(3)гаЛњЮяX(![]() )ЙуЗКДцдкгкЫЎЙћжаЃЌгШвдЦЛЙћЁЂЦЯЬбЁЂЮїЙЯЁЂЩНщЋФкКЌСПНЯЖрЁЃ
)ЙуЗКДцдкгкЫЎЙћжаЃЌгШвдЦЛЙћЁЂЦЯЬбЁЂЮїЙЯЁЂЩНщЋФкКЌСПНЯЖрЁЃ
ЂйгаЛњЮяXжаКЌгаЕФЙйФмЭХУћГЦЪЧ____________________________________ЃЛ
дквЛЖЈЬѕМўЯТЃЌгаЛњЮяXПЩЗЂЩњЛЏбЇЗДгІЕФРраЭга________(ЬюзжФИ)ЁЃ
AЃЎЫЎНтЗДгІ BЃЎШЁДњЗДгІ CЃЎМгГЩЗДгІ DЃЎЯћШЅЗДгІ EЃЎМгОлЗДгІ FЃЎжаКЭЗДгІ
ЂкаДГіXгыН№ЪєФЦЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЃК___________________________________ЁЃ
ЂлгыXЛЅЮЊЭЌЗжвьЙЙЬхЕФЪЧ________(ЬюзжФИ)ЁЃ
aЃЎ![]() bЃЎ
bЃЎ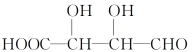
cЃЎ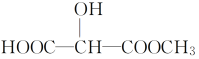 dЃЎ
dЃЎ![]()
eЃЎ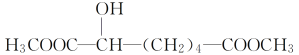 fЃЎ
fЃЎ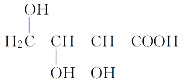
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПШєФГдзгдкДІгкФмСПзюЕЭзДЬЌЪБЃЌЭтЮЇЕчзгХХВМЮЊ![]() ЃЌдђЯТСаЫЕЗЈДэЮѓЕФЪЧЃЈ ЃЉ
ЃЌдђЯТСаЫЕЗЈДэЮѓЕФЪЧЃЈ ЃЉ
A. ИУдЊЫиПЩФмга+3Мл
B. ИУдЊЫиЮЛгкЕк5жмЦкЕкIIIBзх
C. ИУдЊЫидзгКЫЭтЙВга39ИіВЛЭЌдЫЖЏзДЬЌЕФЕчзг
D. ИУдЊЫиЛљЬЌдзгЕкNФмВуЩЯЛЙга5ИіПеЙьЕР
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПНЋ1.92 g CuКЭвЛЖЈСПЕФХЈHNO3ЗДгІЃЌЫцзХCuЕФВЛЖЯМѕЩйЃЌЗДгІЩњГЩЦјЬхЕФбеЩЋж№НЅБфЧГЃЌЕБCuЗДгІЭъБЯЪБЃЌЙВЪеМЏЕНЦјЬх1.12 L(БъзМзДПі)ЃЌдђЗДгІжаЯћКФHNO3ЕФЮяжЪЕФСПЮЊ
A.0.1 molB.0.05 molC.0.15 molD.0.11 mol
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЪЕбщЙ§ГЬжаВњЩњЕФЯжЯѓгыЖдгІЕФЭМаЮЯрЗћЕФЪЧ(ЁЁЁЁ)
A.  NaHSO3 ЗлФЉМгШыHNO3ШмвКжаB.
NaHSO3 ЗлФЉМгШыHNO3ШмвКжаB. 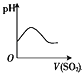 SO2ЦјЬхЭЈШыаТжЦТШЫЎжа
SO2ЦјЬхЭЈШыаТжЦТШЫЎжа
C. 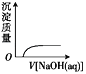 NaOHШмвКЕЮШы Ba(HCO3)2ШмвКжаD.
NaOHШмвКЕЮШы Ba(HCO3)2ШмвКжаD.  CO2ЦјЬхЭЈШыГЮЧхЪЏЛвЫЎжа
CO2ЦјЬхЭЈШыГЮЧхЪЏЛвЫЎжа
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com