
����Ŀ�������Ǹ�ˮ����Ļ�ѧʽΪ[Cr(CH3COO)2]2��2H2O����ˮ����ͨ��Ϊ����ɫ���壬��һ�ֳ��õ��������ռ���������ˮ������(һ���ӷ����л��ܼ�)�������Ҵ������������ᣬ�ױ���������֪Cr3+ˮ��Һ����ɫ��Cr2+ˮ��Һ����ɫ��ʵ�����Ʊ������Ǹ�ˮ�����װ������ͼ��ʾ��

(1)���װ�������Ժ������������ƿ�����μ������п��������CrCl3��Һ���ر�K1��K2������a�����������ƺõ��١�a��������___________����ʱ���������ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ_______��________��һ��ʱ�������װ���ڳ����������������ų������۲쵽���������ƿ����Һ��ɫ����ɫ��ȫת��Ϊ��ɫʱ���ر�K2����K1�������������ƿ�����ɵ�CrCl2��Һѹ���Ҳ�������ƿ�У����Ҳ�������ƿ�з�����Ӧ�����ӷ���ʽΪ________________________________________��
(2)��ʵ��������������Һ��ˮ������У���ԭ����______________________���Ҳ���ջ���ʢ��ˮ������ˮ��������_______________________________________________��
(3)���۲쵽�Ҳ�������ƿ�ڳ��ִ�������ɫ����ʱ���ر�a ��������������ɫ������ٹ��ˡ�ˮϴ������ϴ��������õ�[Cr(CH3COO)2]2��2H2O������������ϴ�Ӳ����Ŀ����_______________________��
(4)�����õ���[Cr(CH3COO)2]2��2H2O���壬����Ϊm g,������ȡ�õ�CrCl3��Һ�к�����n g����[Cr(CH3COO)2]2��2H2O(M1=376 )�IJ�����______%��
���𰸡� ��Һ©�� Zn+2HCl==ZnCl2+H2�� Zn+2CrCl3==ZnCl2+2CrCl2 2Cr2++4CH3COO-+2H2O=[Cr(CH3COO)2]2��2H2O ��ֹˮ�е��ܽ�������Cr2+ ˮ�⣬��ֹ��������װ���� ���ѻӷ�����ˮ�֣�����ʹ��Ʒ���ٸ��� 31700m/376n
��������(1)���������Ľṹ������aΪ��Һ©�������������ƿ���з�����Ӧ�Ļ�ѧ����ʽΪ��2CrCl3+Zn�T2CrCl2+ZnCl2������п��ϡ���ᷴӦ����������Zn+2HCl=ZnCl2+H2����һ��ʱ�������װ���ڳ����������������ų������۲쵽���������ƿ����Һ��ɫ����ɫ��ȫת��Ϊ��ɫʱ���ر�K2����K1�������������ƿ�����ɵ�CrCl2��Һѹ���Ҳ�������ƿ�У�CrCl2��Һ��CH3COONa��Һ��Ӧ���ɴ����Ǹ�ˮ��������[Cr(CH3COO)2]2��2H2O����Ӧ�����ӷ���ʽΪ2Cr2++4CH3COO-+2H2O=[Cr(CH3COO)2]2��2H2O���ʴ�Ϊ����Һ©����Zn+2HCl==ZnCl2+H2����Zn+2CrCl3==ZnCl2+2CrCl2��2Cr2++4CH3COO-+2H2O=[Cr(CH3COO)2]2��2H2O��
(2) ��ʵ�������õ���Һ�������õ�����ˮ����������У�ԭ���ǣ����۸����ȶ������ױ�����������ȥ��ˮ�е��ܽ�������ֹCr2+���������Ҳ��ջ��ڵ�ˮ���Է�ֹ��������װ���ڣ��ʴ�Ϊ����ֹˮ�е��ܽ�������Gr2+��ˮ�⣬��ֹ��������װ����
(3)���۲쵽�Ҳ�������ƿ�ڳ��ִ�������ɫ����ʱ���ر�a��������������ɫ������ٹ��ˡ�ˮϴ������ϴ��������õ�[Cr(CH3COO)2]2��2H2O������������ϴ�ӣ����ѻӷ�����ˮ�֣�����ʹ��Ʒ���ٸ���ʴ�Ϊ�����ѻӷ�����ˮ�֣�����ʹ��Ʒ���ٸ��
(4) ʵ��ʱȡ�õ�CrCl3��Һ�к�����ng�������ϵõ�[Cr(CH3COO)2]22H2O������=![]() ��
��![]() ��376g/mol=
��376g/mol=![]() g����ʵ�����ò�Ʒ�IJ���=
g����ʵ�����ò�Ʒ�IJ���=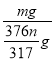 ��100%=
��100%=![]() ��100%=
��100%=![]() %���ʴ�Ϊ��
%���ʴ�Ϊ�� ![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������̼��������ϳɻ���ԭ�ϣ��ȿ��Լ��ٻ�����Ⱦ������ЧӦ�����ܱ��Ϊ����
��CO2���ۺ������ǽ������ЧӦ����Դ�������Ч;����
(1)O2��H2�ڴ��������¿ɷ�����Ӧ����CH3OH����֪CH3OH��H2��ȼ���ȷֱ�Ϊ��H1=-akJ��mol-1����H2=-bkJ��mol-1����1molˮ����ת��ΪҺ̬ˮʱ�ų�ckJ��������
��CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)��H=___________kJ��mol-1��
CH3OH(g)+H2O(g)��H=___________kJ��mol-1��
(2)����CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)������CO2��H2��ʼͶ�ϱ�Ϊ1��3ʱ���¶ȶ�CO2ƽ��ת���ʼ��״����ʵ�Ӱ����ͼ��ʾ����ͼ��֪��ȡCH3OH�����˵��¶���___________���������������CO2ת��ΪCH3OH��ƽ��ת���ʵĴ�ʩ��___________��
CH3OH(g)+H2O(g)������CO2��H2��ʼͶ�ϱ�Ϊ1��3ʱ���¶ȶ�CO2ƽ��ת���ʼ��״����ʵ�Ӱ����ͼ��ʾ����ͼ��֪��ȡCH3OH�����˵��¶���___________���������������CO2ת��ΪCH3OH��ƽ��ת���ʵĴ�ʩ��___________��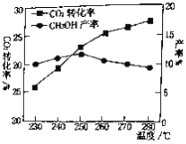
A��ʹ�ô��� B��������ϵѹǿ
C������CO2��H2�ij�ʼͶ�ϱ� D��Ͷ�ϱȲ��������������䣬���ӷ�Ӧ���Ũ��
��CO�Ǻϳ����ء������ԭ�ϡ�
(3)�ϳ����صķ�Ӧ��2NH3(g)+CO(g)![]() CO(NH2)2(g)+H2(g)��H=-81.0kJ��mol-1��
CO(NH2)2(g)+H2(g)��H=-81.0kJ��mol-1��
��T��ʱ�������Ϊ2L�ĺ����ܱ������У���2molNH3��1molCO��Ϸ�����Ӧ��5minʱ��NH3��ת����Ϊ80%����0��5min�ڵ�ƽ����Ӧ����Ϊv(CO)=___________��
����֪��
�¶�/K | 398 | 498 | �� |
ƽ�ⳣ��/K | 126.5 | K1 | �� |
��K1___________126.5(�������<��);���������___________��
(4)ͨ���˹�������ÿɽ�COת����HCOOH��
����֪�����£�Ũ�Ⱦ�Ϊ0.1mol��L-1��HCOOH��HCOONa�����ҺpH=3.7,��HCOOH�ĵ��볣��Ka��ֵΪ___________ (��֪lg2=0.3)��
���õ绯ѧ������HCOOH��ˮ����ɵ���Ⱦ����ԭ���ǵ��CoSO4��ϡ�����HCOOH�����Һ���õ�������Co3+��HCOOH������CO2��Co3+����HCOOH�����ӷ���ʽΪ___________;��������仯�����ǰ��Co2+��Ũ�Ƚ�___________ (�������С�����䡱)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Һ��ʯ�����Ǿ���ѹ��ѹ������ƿ�еģ�ƿ��ѹǿ�Ǵ���ѹǿ��7-8����Һ��ʯ��������Ҫ�ɷ��DZ��顢���顢��ϩ�Ͷ�ϩ�ȡ������йض����������ȷ���ǣ� ��

A. ������̼����Ԫ�صĸ�����Ϊ2��5
B. �����������Ԫ�ص������������
C. ��������̼���⡢��ԭ�ӹ��ɵ��л���
D. ��ͨ��״���£�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ƭ����������Һ�У���Ƭ�ܽ⣬��Һ�������ӣ���û������ų����ǣ� ��
A.ϡ����
B.CuSO4��Һ
C.Fe2��SO4��3��Һ
D.AgNO3��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Na2SO3��һ����Ҫ�Ļ�ԭ����I2O5��һ����Ҫ�������������߶��ǻ�ѧʵ�����е���Ҫ�Լ���
(1)��֪:2Na2SO3 (aq)+O2(aq)==2Na2SO4(aq) ��H =m kJ��mol-1��O2(g)![]() O2(aq) ��H =n kJ��mol-1 ����Na2SO3��Һ��O2(g)��Ӧ���Ȼ�ѧ����ʽΪ______________________________��
O2(aq) ��H =n kJ��mol-1 ����Na2SO3��Һ��O2(g)��Ӧ���Ȼ�ѧ����ʽΪ______________________________��
(2)Na2SO3�������ָ�������ƶ���������Σ�ƶ�������ʷ���Ϊv=k��ca(SO32-)��cb(O2)��kΪ������
�ٵ��ܽ���Ũ��Ϊ4.0 mg/L(��ʱNa2SO3������λ��ƶ����)ʱ��c(SO32-)��������ֵ��ϵ���±���ʾ����a=____��
c(SO32-)��103 | 3.65 | 5.65 | 7.65 | 11.65 |
V��106 | 10.2 | 24.4 | 44.7 | 103.6 |
�������ε����ʷ��̺Ͳ�ͬ�¶ȵ����ʳ���֮�����±���ʾ����֪1n(k2/k1)=Ea/R(1/T2-1/T1)��R Ϊ��������Ea(������)______(�>����<��)Ea(ƶ����)��
��Ӧ�� | ���ʷ��� | k(297.0K)/k(291.5K) |
������ | v= k��c(SO32-)��c(O2) | 1.47 |
ƶ���� | v= k��ca(SO32-)��cb(O2) | 2.59 |
(3)�����ʵ�����Na2SO3��Na2SO4�����Һ�У�c(SO32-) +c( HSO3-)______(�>����<����=��)c(SO42-)��
(4)����I2O5������CO ��Ⱦ���䷴ӦΪI2O5(s)+5CO(g) ![]() 5CO2(g)+I2(s)����ͬ�¶��£���װ������I2O5�����2 L �����ܱ�������ͨ��2 mol CO�����CO2��������������(CO2) ��ʱ��t�ı仯������ͼ��ʾ��
5CO2(g)+I2(s)����ͬ�¶��£���װ������I2O5�����2 L �����ܱ�������ͨ��2 mol CO�����CO2��������������(CO2) ��ʱ��t�ı仯������ͼ��ʾ��

�ٴӷ�Ӧ��ʼ��a��ʱ��ƽ����Ӧ����v(CO)=__________��
��b��ʱ��CO ��ת����Ϊ_____________��
��b�� �� d�� �� ��ѧ ƽ�ⳣ��:Kb____(� >����<����=�� )Kd���жϵ�������_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2008��ŵ������ѧ��������������Ǯ��������λ��ѧ�ң��Ա������Ƿ��ֺ��о�����ɫӫ�⵰�ס�ӫ�����Ƿ������ʵĻ��ʣ� 5-�Ȼ�ӫ������5-�Ȼ�������ӫ�����ڼ�����������ǿ�ҵ���ɫӫ�⣬���ǹ㷺Ӧ����ӫ������ȡ�

![]()
����˵������ȷ����
A. 5-FAMת��Ϊ5-CFDA����ȡ����Ӧ
B. 5-FAM�ķ���ʽΪC21H12O7
C. 5-FAM��5-CFDA ��1mol�ֱ�������NaOH��Һ��Ӧ������NaOH ���ʵ�����ͬ
D. ʵ���Ҽ���5-FAM��5-CFDA����NaHCO3 ��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ����գ�
��1��3.6gH2O���ʵ���Ϊ______mol��Լ����_________��ԭ�ӣ�
��2����֪1.204��1023��X�����������6.4g����X�����Ħ��������______��
��3��ͬ��ͬѹ�£�ͬ����ļ���CH4��CO2����֮��Ϊ________��������֮��_________��
��4��VLAl2��SO4��3��Һ�к�Al3+amol����Al2��SO4��3��Һ�����ʵ���Ũ��Ϊ__________��ȡ��V/2L�ټ���ˮϡ�͵�4VL����ϡ�ͺ���Һ��SO42-�����ʵ���Ũ����__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��
��������ָ�ܽ����������̵�Ļ�ѧƷ��һ���Ǹ߷����л������������I���������ʼӾ۵õ��ĸ߷��ӻ����ͨ��������Ʒ���Խ������̵㣬����ȼ����Ʒ��ʹ�÷�Χ��Ϊ�ϳɵõ�������I���������ϩ��AΪ��ʼԭ�ϵĺϳ�·�ߣ�

�����
(1) A�����ƣ�________________��B��C�ķ�Ӧ������____________________��
F�к��еĹ��������ƣ�______________________��
(2) E������һ�������ºϳɸ߾���J��д����Ӧ�Ļ�ѧ����ʽ��_______________________��
(3) H��һ�ֺ˴Ź�������һ������Ԫ��״�ṹ��д��������I�Ľṹ��ʽ��____________��
(4) F�ж���ͬ���칹�壬���Т�����NaOH��Һ��Ӧ��������NaHCO3��Һ��Ӧ����ʹ��ˮ��ɫ��ͬ���칹����______��(������˳���칹)��д�����к˴Ź����������֮Ϊ1��1��2��2��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��____________________________________��
(5) ���Ϻϳ�·��д���ɱ��Ҵ����Ҵ�Ϊԭ�Ϻϳ� �������������___________________ (�������Լ���ѡ)
�������������___________________ (�������Լ���ѡ)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com