
����Ŀ��
��������ָ�ܽ����������̵�Ļ�ѧƷ��һ���Ǹ߷����л������������I���������ʼӾ۵õ��ĸ߷��ӻ����ͨ��������Ʒ���Խ������̵㣬����ȼ����Ʒ��ʹ�÷�Χ��Ϊ�ϳɵõ�������I���������ϩ��AΪ��ʼԭ�ϵĺϳ�·�ߣ�

�����
(1) A�����ƣ�________________��B��C�ķ�Ӧ������____________________��
F�к��еĹ��������ƣ�______________________��
(2) E������һ�������ºϳɸ߾���J��д����Ӧ�Ļ�ѧ����ʽ��_______________________��
(3) H��һ�ֺ˴Ź�������һ������Ԫ��״�ṹ��д��������I�Ľṹ��ʽ��____________��
(4) F�ж���ͬ���칹�壬���Т�����NaOH��Һ��Ӧ��������NaHCO3��Һ��Ӧ����ʹ��ˮ��ɫ��ͬ���칹����______��(������˳���칹)��д�����к˴Ź����������֮Ϊ1��1��2��2��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��____________________________________��
(5) ���Ϻϳ�·��д���ɱ��Ҵ����Ҵ�Ϊԭ�Ϻϳ� �������������___________________ (�������Լ���ѡ)
�������������___________________ (�������Լ���ѡ)
���𰸡� ��ϩ NaOHˮ��Һ���� ̼̼˫�����Ȼ� 
 5 HCOOCH2CH=CH2+NaOH��HCOONa+HOCH2CH=CH2
5 HCOOCH2CH=CH2+NaOH��HCOONa+HOCH2CH=CH2 
�������������������G�����Ƴ�FΪ �����������Ϣ
�����������Ϣ ��E��F�ķ�Ӧ��������F�Ƴ�EΪ
��E��F�ķ�Ӧ��������F�Ƴ�EΪ �����C��D�ķ�Ӧ����������E�Ƴ�DΪ��ͪ��CH3COCH3����CΪ2-������BΪ2-�ȱ��顢AΪ��ϩ��
�����C��D�ķ�Ӧ����������E�Ƴ�DΪ��ͪ��CH3COCH3����CΪ2-������BΪ2-�ȱ��顢AΪ��ϩ��
(1) A������Ϊ��ϩ��B��C�ķ�Ӧ��2-�ȱ���ˮ�⣬��Ӧ�����ǣ�NaOHˮ��Һ������
F�к��еĹ�������̼̼˫�����Ȼ���
(2) E�����м����ǻ������Ȼ�����һ�������·������۷�Ӧ���ɸ߷���������Ӧ�Ļ�ѧ����ʽΪ�� ��
��
(3) H�ķ���ʽ��C4H2O3����һ�ֺ˴Ź�������һ������Ԫ��״�ṹ����H�Ľṹ��ʽΪ ��H����G�����Ӿ۷�Ӧ���ɽ�����I������I�Ľṹ��ʽΪ
��H����G�����Ӿ۷�Ӧ���ɽ�����I������I�Ľṹ��ʽΪ ��
��
(4) F�� ���ж���ͬ���칹�壬���Т�����NaOH��Һ��Ӧ��������NaHCO3��Һ��Ӧ��˵�� ������������������ʹ��ˮ��ɫ��˵����������̼̼˫��������������ͬ���칹����HCOOCH2CH=CH2��HCOO CH=CHCH3��HCOOC (CH3)=CH2��CH3COOCH=CH2��CH2= CH COOCH3����5�֣����к˴Ź����������֮Ϊ1��1��2��2��NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ��HCOOCH2CH=CH2+NaOH��HCOONa+HOCH2CH=CH2��
���ж���ͬ���칹�壬���Т�����NaOH��Һ��Ӧ��������NaHCO3��Һ��Ӧ��˵�� ������������������ʹ��ˮ��ɫ��˵����������̼̼˫��������������ͬ���칹����HCOOCH2CH=CH2��HCOO CH=CHCH3��HCOOC (CH3)=CH2��CH3COOCH=CH2��CH2= CH COOCH3����5�֣����к˴Ź����������֮Ϊ1��1��2��2��NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ��HCOOCH2CH=CH2+NaOH��HCOONa+HOCH2CH=CH2��
(5)�ɱ��Ҵ����Ҵ�Ϊԭ�Ϻϳ� �������Ȱѱ��Ҵ�����Ϊ����ȩ��Ȼ�������Ϣ
�������Ȱѱ��Ҵ�����Ϊ����ȩ��Ȼ�������Ϣ ���ѱ���ȩת��Ϊ
���ѱ���ȩת��Ϊ ��
�� �ٷ�����ȥ��Ӧ����
�ٷ�����ȥ��Ӧ����![]() ��
��![]() ���Ҵ�����������Ӧ����
���Ҵ�����������Ӧ����![]() ��
��![]() �����Ӿ۷�Ӧ�õ���Ʒ
�����Ӿ۷�Ӧ�õ���Ʒ ������ϳ�·�����£�
������ϳ�·�����£� 


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ǹ�ˮ����Ļ�ѧʽΪ[Cr(CH3COO)2]2��2H2O����ˮ����ͨ��Ϊ����ɫ���壬��һ�ֳ��õ��������ռ���������ˮ������(һ���ӷ����л��ܼ�)�������Ҵ������������ᣬ�ױ���������֪Cr3+ˮ��Һ����ɫ��Cr2+ˮ��Һ����ɫ��ʵ�����Ʊ������Ǹ�ˮ�����װ������ͼ��ʾ��

(1)���װ�������Ժ������������ƿ�����μ������п��������CrCl3��Һ���ر�K1��K2������a�����������ƺõ��١�a��������___________����ʱ���������ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ_______��________��һ��ʱ�������װ���ڳ����������������ų������۲쵽���������ƿ����Һ��ɫ����ɫ��ȫת��Ϊ��ɫʱ���ر�K2����K1�������������ƿ�����ɵ�CrCl2��Һѹ���Ҳ�������ƿ�У����Ҳ�������ƿ�з�����Ӧ�����ӷ���ʽΪ________________________________________��
(2)��ʵ��������������Һ��ˮ������У���ԭ����______________________���Ҳ���ջ���ʢ��ˮ������ˮ��������_______________________________________________��
(3)���۲쵽�Ҳ�������ƿ�ڳ��ִ�������ɫ����ʱ���ر�a ��������������ɫ������ٹ��ˡ�ˮϴ������ϴ��������õ�[Cr(CH3COO)2]2��2H2O������������ϴ�Ӳ����Ŀ����_______________________��
(4)�����õ���[Cr(CH3COO)2]2��2H2O���壬����Ϊm g,������ȡ�õ�CrCl3��Һ�к�����n g����[Cr(CH3COO)2]2��2H2O(M1=376 )�IJ�����______%��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ������ȷ��ӳ��Ӧ�仯��ϵ����( )
A. ���ȸ�����ع�����ȡ����
B. ����һ��Ũ�ȵ�����������Һ��ˮϡ��
C. ����ij�¶��µĽӽ����͵��������Һ����������ع���
D. ��һ��Ũ�ȵ�ϡ�����м����������þ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ƿ�չ�е�����Դ���������ð�������Ʊ��������Ӧ���������ڡ��ش�����������
��1����������ȣ�������Ϊȼ�ϵ��ŵ���______________�����ٴ������������������ֱ��ȼ�յ�������ת����Զ����ȼ�ϵ�أ�д����������ȼ�ϵ�صĸ�����Ӧʽ��_____________________________��
��2�������������Ʊ�H2O2����֪��H2(g)+A(l)![]() B(l)��H1 O2(g)+B(l)
B(l)��H1 O2(g)+B(l)![]() A(l)+H2O2(l) ��H2������A��BΪ�л������Ӧ��Ϊ�Է���Ӧ����H2(g)+ O2(g)
A(l)+H2O2(l) ��H2������A��BΪ�л������Ӧ��Ϊ�Է���Ӧ����H2(g)+ O2(g)![]() H2O2(l)�Ħ�H____0(�>������<����=��)��
H2O2(l)�Ħ�H____0(�>������<����=��)��
��3���ں��º��ݵ��ܱ������У�ij���ⷴӦ��MHx(s)+yH2(g)=MHx+2y(s) ��H<0�ﵽ��ѧƽ�⡣�����й�������ȷ����________��
a.����������ѹǿ���ֲ��� b.����y mol H2ֻ��1 mol MHx
c.�����£��÷�Ӧ��ƽ�ⳣ������ d.����������ͨ��������������v(����)>v(����)
��4������̫����ֱ�ӷֽ�ˮ���⣬�����������������;����������ת����ʽΪ_______��
��5�����������ĸ�����Ҳ����������Դ����ⷨ��ȡ�й㷺��;��Na2FeO4��ͬʱ���������Fe+2H2O+2OH![]() FeO42+3H2��������ԭ����ͼ1��ʾ��װ��ͨ������缫���������Ϻ�ɫ��FeO42�����缫�����ݲ�����������������ҺŨ�ȹ��ߣ����缫����������ɫ���ʡ���֪��Na2FeO4ֻ��ǿ�����������ȶ����ױ�H2��ԭ��
FeO42+3H2��������ԭ����ͼ1��ʾ��װ��ͨ������缫���������Ϻ�ɫ��FeO42�����缫�����ݲ�����������������ҺŨ�ȹ��ߣ����缫����������ɫ���ʡ���֪��Na2FeO4ֻ��ǿ�����������ȶ����ױ�H2��ԭ��

�ٵ��һ��ʱ���c(OH-)���͵�������_______��������ҡ��������ҡ�����
�ڵ������У��뽫�������������弰ʱ�ų�����ԭ����________________��
��c(Na2FeO4)���ʼc(NaOH)�ı仯��ͼ2����ѡM��N�����е�һ�㣬����c(Na2FeO4)�������ֵ��ԭ��_______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������(K2FeO4)�������ˮ�����������к�ǿ�������ԣ������������������ͳ���������ˮ������Чɱ��ˮ�е���������࣬���������ֽ�����л�������Ⱦ���ӡ��л���������軯��ȣ��������������������в���������ȼ��顢�ȴ��ӵȶ�����Ⱦ���ҵ�ϳ��ô��������������͵�ⷨ���Ƶø������ƺ������������ر�����Һ��Ӧ�Ʊ�������ء�
I.��ⷨ���������������������Һ����˿����ʯī���缫���ϣ����Ƶø������ƣ����ж���˿����_______���� ��������������__________��
II.���������������Ʊ�������ؼ�Ҫ�������£�

(1)д���ڼ��������¹��̢ٷ�Ӧ�����ӷ���ʽ��___________________________________��
(2)���̢ڽ������Һ�����Сʱ�����ã����˻�ôֲ�Ʒ���÷�Ӧ�Ļ�ѧ����ʽΪ��2KOH+Na2FeO4��K2FeO4+2NaOH������ݸ��ֽⷴӦԭ��������Ӧ������ԭ��______________________________________��
(3)K2FeO4����ˮ�������ų����壬��ɱ������������ˮ���������ʣ�д������ˮ��Ӧ�����ӷ���ʽ_______________________________________________________�����ᴿK2FeO4ʱ�����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ��___________������ţ���
A��H2O B��ϡKOH��Һ�������
C��KCl��Һ������� D��Fe(NO3)3��Һ�������
(4)K2FeO4����ˮ��ˮ����������ͼ�е����֣��������ʾ��ֲ�������������˵������ȷ����________________��
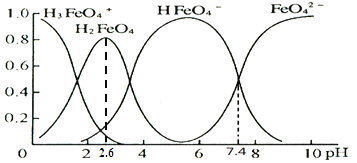
A��FeO42+H2OHFeO4+OH��ƽ�ⳣ��Ϊ107.4
B����pH=1��pH=2.6��H2FeO4�ķֲ�����������
C����pH=6�ĸ���Һ�м�KOH��Һ����Ӧ�����ӷ���ʽΪHFeO4+ OH = FeO42+H2O
D������ͼ����Ϣ����Ԫ����4�ִ�����̬���������ǿ���ͬʱ��������
(5)���õζ��������ⶨ��K2FeO4�Ĵ��ȣ��йط�Ӧ���ӷ���ʽΪ��
��FeO42+CrO2 +2H2O=CrO42+Fe(OH)3��+OH
��2CrO42+2H+=Cr2O72+H2O
��Cr2O72+6Fe2++14H+=2Cr3++6Fe3+ +7H2O
�ֳ�ȡ1.980g�ָ��������Ʒ������������������Һ�У������Թ�����KCrO2����ַ�Ӧ����ˣ���Һ������250 mL����ƿ�С�ÿ��ȡ25.00 mL����ϡ�����ữ����0.1000 mol/L��(NH4)2Fe(SO4)2����Һ�ζ������εζ����ı���Һ��ƽ�����Ϊ21.90 mL����������Ʒ�и�����ص���������Ϊ______________����������λ��Ч���֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
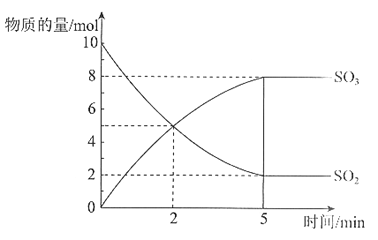
����Ŀ����2 L���ܱ������У�SO2��O2�ڴ���500��������·�����Ӧ��SO2��SO3�����ʵ�����ʱ��仯�Ĺ�ϵ������ͼ��ʾ��
�ش��������⡣
��1���÷�Ӧ�Ļ�ѧ����ʽ��_________��
��2����ǰ2 min�ڣ���SO2��Ũ�ȱ仯��ʾ��������_________mol����L��min����
��3����Ӧ�ﵽƽ��״̬��������_________��
a. ��λʱ��������1 mol SO2��ͬʱ����1 mol SO3
b. SO2��Ũ����SO3Ũ�Ⱦ����ٱ仯
c. SO2��Ũ����SO3Ũ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ����ѧ��ѧ�г����ڻ����ķ�����ᴿ��װ�ã������װ�ûش����⣺

��1����װ��A��װ��B�ж��õ���������װ��A�в�������������__________��װ��B�в�������������__________����ֹ����������Һ��ֲ����ȶ�������
��2��װ��C�Тٵ�������__________�����м����ʯ��������______________����ȴˮ�ķ�����_________��װ��D�ڷ�ҺʱΪʹҺ��˳�����£�Ӧ���еľ��������_________��
��3�����Ȼ�����Һ�еõ��Ȼ��ƹ��壬ѡ��װ��__________(�����װ��ͼ����ĸ����ͬ)����ȥ����ˮ�е�Cl-�����ʣ�ѡ��װ��__________����������ˮ��Cl-�Ƿ�����ķ�����ȡ������ƿ�е�ˮ�ڽྻ�Թ��У��μ�________����������ɫ��������Cl-�ѳ�����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com