
����Ŀ���������(K2FeO4)�������ˮ�����������к�ǿ�������ԣ������������������ͳ���������ˮ������Чɱ��ˮ�е���������࣬���������ֽ�����л�������Ⱦ���ӡ��л���������軯��ȣ��������������������в���������ȼ��顢�ȴ��ӵȶ�����Ⱦ���ҵ�ϳ��ô��������������͵�ⷨ���Ƶø������ƺ������������ر�����Һ��Ӧ�Ʊ�������ء�
I.��ⷨ���������������������Һ����˿����ʯī���缫���ϣ����Ƶø������ƣ����ж���˿����_______���� ��������������__________��
II.���������������Ʊ�������ؼ�Ҫ�������£�

(1)д���ڼ��������¹��̢ٷ�Ӧ�����ӷ���ʽ��___________________________________��
(2)���̢ڽ������Һ�����Сʱ�����ã����˻�ôֲ�Ʒ���÷�Ӧ�Ļ�ѧ����ʽΪ��2KOH+Na2FeO4��K2FeO4+2NaOH������ݸ��ֽⷴӦԭ��������Ӧ������ԭ��______________________________________��
(3)K2FeO4����ˮ�������ų����壬��ɱ������������ˮ���������ʣ�д������ˮ��Ӧ�����ӷ���ʽ_______________________________________________________�����ᴿK2FeO4ʱ�����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ��___________������ţ���
A��H2O B��ϡKOH��Һ�������
C��KCl��Һ������� D��Fe(NO3)3��Һ�������
(4)K2FeO4����ˮ��ˮ����������ͼ�е����֣��������ʾ��ֲ�������������˵������ȷ����________________��
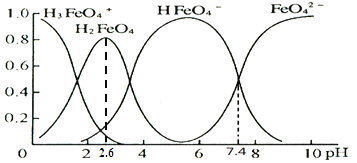
A��FeO42+H2OHFeO4+OH��ƽ�ⳣ��Ϊ107.4
B����pH=1��pH=2.6��H2FeO4�ķֲ�����������
C����pH=6�ĸ���Һ�м�KOH��Һ����Ӧ�����ӷ���ʽΪHFeO4+ OH = FeO42+H2O
D������ͼ����Ϣ����Ԫ����4�ִ�����̬���������ǿ���ͬʱ��������
(5)���õζ��������ⶨ��K2FeO4�Ĵ��ȣ��йط�Ӧ���ӷ���ʽΪ��
��FeO42+CrO2 +2H2O=CrO42+Fe(OH)3��+OH
��2CrO42+2H+=Cr2O72+H2O
��Cr2O72+6Fe2++14H+=2Cr3++6Fe3+ +7H2O
�ֳ�ȡ1.980g�ָ��������Ʒ������������������Һ�У������Թ�����KCrO2����ַ�Ӧ����ˣ���Һ������250 mL����ƿ�С�ÿ��ȡ25.00 mL����ϡ�����ữ����0.1000 mol/L��(NH4)2Fe(SO4)2����Һ�ζ������εζ����ı���Һ��ƽ�����Ϊ21.90 mL����������Ʒ�и�����ص���������Ϊ______________����������λ��Ч���֣�
���𰸡� I���� H2 2Fe3++3ClO+10OH=2FeO42+3Cl+5H2O ���¶���K2FeO4�ܽ��С��Na2FeO4 4FeO42+10H2O=4Fe(OH)3+3O2+8OH B AD 73.00%
��������I.��ⷨ���������������������Һ����˿����ʯī���缫���ϣ����Ƶø������ơ����ڷ�Ӧ�б�����Ϊ�����������˿��һ���������������ϣ������ӷŵ������������
II.(1)�ڼ��������¹��̢ٷ�Ӧ�����ӷ���ʽΪ2Fe3++3ClO+10OH=2FeO42+3Cl+5H2O��
(2)���̢ڷ�����Ӧ�Ļ�ѧ����ʽΪ��2KOH+Na2FeO4��K2FeO4+2NaOH���÷�Ӧ֮�����ܷ���������Ϊ�ڷ�Ӧ���Ƶ��¶���K2FeO4�ܽ��С��Na2FeO4��
(3)K2FeO4����ˮ�������ų����壬��ɱ������������ˮ���������ʣ��ɴ˿�֪���÷�Ӧ���������������������壬�÷�Ӧ�����ӷ���ʽΪ4FeO42+10H2O=4Fe(OH)3+3O2+8OH�����ᴿK2FeO4ʱ�����ؽᾧ��ϴ�ӡ����º�ɵķ�����Ϊ���پ�����ܽ���ʧ����ֹ�����µ����ʣ����ڵ��º�ɣ�ϴ�Ӽ����ѡ��ϡKOH��Һ���������ѡB��
(4) A����ͼ��֪��pH=7.4ʱ����c(H+)=10-7.4mol/L��c(OH-)=10-6.6mol/L��c(FeO42)=c(HFeO4)��������FeO42+H2OHFeO4+OH��ƽ�ⳣ���ı���ʽ��֪���÷�Ӧ��ƽ�ⳣ��Ϊ106.6 ��A����ȷ��B����ͼ��֪����pH=1��pH=2.6��H2FeO4�ķֲ�������������B��ȷ��C����ͼ��֪��pHԽ��HFeO4�ķֲ�����ԽС��FeO42�ķֲ�����Խ�����ԣ���pH=6�ĸ���Һ�м�KOH��Һ����Ӧ�����ӷ���ʽΪHFeO4+ OH = FeO42+H2O��C��ȷ��D����ͼ��֪��pH��Сʱ�������������������Ӻ������٣����Ƕ����������ӣ���pH�ܴ�ʱ�������������������Ӻ������٣����Ƕ������������ӣ����������Dz���������ͬʱ�������ڡ�����������˵������ȷ����AD��
(5)�õζ��������ⶨ��K2FeO4�Ĵ��ȣ��йط�Ӧ���ӷ���ʽΪ��
��FeO42+CrO2+2H2O=CrO42+Fe(OH)3��+OH
��2CrO42+2H+=Cr2O72+H2O
��Cr2O72+6Fe2++14H+=2Cr3++6Fe3+ +7H2O
�ɴ˿ɵù�ϵʽ2FeO42~2CrO2~ 2CrO42~ Cr2O72~6Fe2+������n(FeO42)=![]() n(Fe2+)=
n(Fe2+)= ![]() =7.300
=7.300![]() mol��������Ʒ�и�����ص���������Ϊ
mol��������Ʒ�и�����ص���������Ϊ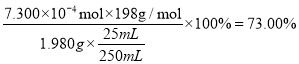 ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Na2SO3��һ����Ҫ�Ļ�ԭ����I2O5��һ����Ҫ�������������߶��ǻ�ѧʵ�����е���Ҫ�Լ���
(1)��֪:2Na2SO3 (aq)+O2(aq)==2Na2SO4(aq) ��H =m kJ��mol-1��O2(g)![]() O2(aq) ��H =n kJ��mol-1 ����Na2SO3��Һ��O2(g)��Ӧ���Ȼ�ѧ����ʽΪ______________________________��
O2(aq) ��H =n kJ��mol-1 ����Na2SO3��Һ��O2(g)��Ӧ���Ȼ�ѧ����ʽΪ______________________________��
(2)Na2SO3�������ָ�������ƶ���������Σ�ƶ�������ʷ���Ϊv=k��ca(SO32-)��cb(O2)��kΪ������
�ٵ��ܽ���Ũ��Ϊ4.0 mg/L(��ʱNa2SO3������λ��ƶ����)ʱ��c(SO32-)��������ֵ��ϵ���±���ʾ����a=____��
c(SO32-)��103 | 3.65 | 5.65 | 7.65 | 11.65 |
V��106 | 10.2 | 24.4 | 44.7 | 103.6 |
�������ε����ʷ��̺Ͳ�ͬ�¶ȵ����ʳ���֮�����±���ʾ����֪1n(k2/k1)=Ea/R(1/T2-1/T1)��R Ϊ��������Ea(������)______(�>����<��)Ea(ƶ����)��
��Ӧ�� | ���ʷ��� | k(297.0K)/k(291.5K) |
������ | v= k��c(SO32-)��c(O2) | 1.47 |
ƶ���� | v= k��ca(SO32-)��cb(O2) | 2.59 |
(3)�����ʵ�����Na2SO3��Na2SO4�����Һ�У�c(SO32-) +c( HSO3-)______(�>����<����=��)c(SO42-)��
(4)����I2O5������CO ��Ⱦ���䷴ӦΪI2O5(s)+5CO(g) ![]() 5CO2(g)+I2(s)����ͬ�¶��£���װ������I2O5�����2 L �����ܱ�������ͨ��2 mol CO�����CO2��������������(CO2) ��ʱ��t�ı仯������ͼ��ʾ��
5CO2(g)+I2(s)����ͬ�¶��£���װ������I2O5�����2 L �����ܱ�������ͨ��2 mol CO�����CO2��������������(CO2) ��ʱ��t�ı仯������ͼ��ʾ��

�ٴӷ�Ӧ��ʼ��a��ʱ��ƽ����Ӧ����v(CO)=__________��
��b��ʱ��CO ��ת����Ϊ_____________��
��b�� �� d�� �� ��ѧ ƽ�ⳣ��:Kb____(� >����<����=�� )Kd���жϵ�������_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��
A. ��������������Һʱ�������м�����������������
B. ��������ﮱ�����ú����
C. ����Һ�����ˮ��棬��ֹ��ӷ�
D. Ũ�����ô���������ϸ�ڡ���ɫ�Լ�ƿʢ�ţ���������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��H2C2O4Ϊ��Ԫ���ᣮ20��ʱ������һ��c(H2C2O4)+c(HC2O4-)+c(C2O42-)=0.100molL-1��H2C2O4��NaOH�����Һ����Һ�в����������ʵ���Ũ����pH�ı仯������ͼ��ʾ������ָ����Һ���������ʵ���Ũ�ȹ�ϵһ����ȷ���ǣ� ��

A. pH=2.5����Һ�У�c(H2C2O4)+c(C2O42-)��c(HC2O4-)
B. c(Na+)=0.100 molL-1����Һ�У�c(H+)+c(H2C2O4)=c(OH-)+c(C2O42-)
C. c(HC2O4-)=c(C2O42-)����Һ�У�c(Na+)��0.100 molL-1+c(HC2O4-)
D. pH=7����Һ�У�c(Na+)=2c(C2O42-)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����( )
A.pH��5.6��7.0֮�����ˮͨ����Ϊ����
B.������ˮ������һ��ʱ���pH����
C.ȼúʱ��������ʯ��ʯ���ɼ��ٷ�����SO2����
D.������SO2����Ҫ��Դ�������ų���β��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��
��������ָ�ܽ����������̵�Ļ�ѧƷ��һ���Ǹ߷����л������������I���������ʼӾ۵õ��ĸ߷��ӻ����ͨ��������Ʒ���Խ������̵㣬����ȼ����Ʒ��ʹ�÷�Χ��Ϊ�ϳɵõ�������I���������ϩ��AΪ��ʼԭ�ϵĺϳ�·�ߣ�

�����
(1) A�����ƣ�________________��B��C�ķ�Ӧ������____________________��
F�к��еĹ��������ƣ�______________________��
(2) E������һ�������ºϳɸ߾���J��д����Ӧ�Ļ�ѧ����ʽ��_______________________��
(3) H��һ�ֺ˴Ź�������һ������Ԫ��״�ṹ��д��������I�Ľṹ��ʽ��____________��
(4) F�ж���ͬ���칹�壬���Т�����NaOH��Һ��Ӧ��������NaHCO3��Һ��Ӧ����ʹ��ˮ��ɫ��ͬ���칹����______��(������˳���칹)��д�����к˴Ź����������֮Ϊ1��1��2��2��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��____________________________________��
(5) ���Ϻϳ�·��д���ɱ��Ҵ����Ҵ�Ϊԭ�Ϻϳ� �������������___________________ (�������Լ���ѡ)
�������������___________________ (�������Լ���ѡ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и������ʣ�����������ʡ������˳�����е��� �� ��
A.��ʯ�ҡ���ˮ��Ư��B.�ռҺ�ȡ���ˮ
C.�ɱ����֡�����D.���ᡢ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ȷ����
A. c(NH4+)��ȵ�NH4Fe(SO4)2��Һ��NH4HCO3��Һ��(NH4)2SO4��Һ������Ũ�ȴ�С��ϵ����c[(NH4)2SO4] <c[NH4HCO3] <c[NH4Fe(SO4)2]
B. �������£�0.1mol/LHC1��Һ������0.1mol/LBa(OH)2��Һ��Ϻ���Һ��pH=13
C. 0.lmol/L��NaHCO3 ��Һ�У�c(H+)+c(H2CO3)=c(CO32-)+c(OH- )
D. �ö��Ե缫��ⱥ���Ȼ�����Һ: 2C1-+2H+![]() H2��+ Cl2��
H2��+ Cl2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڴ����ᴿ��ʵ���У�����ʱ��ȷ�IJ����ǣ� ��
A.�ѻ��ǵ�Һ�嵹���������ڼ���
B.��ʼ����������ò���������
C.��ˮ����ȫ���ɺ�ֹͣ����
D.�������г��ִ�������ʱ��ֹͣ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com