
����Ŀ����.��Ҫ��д�����з�Ӧ���Ȼ�ѧ����ʽ��
(1)��25����101kPa�£�1g�״�Һ��ȼ������C02��Һ̬ˮʱ����22.0kJ�����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ__________________________��
(2)��������N2��02��ȫ��Ӧ��ÿ����23g N02��Ҫ����16.0kJ�������˷�Ӧ���Ȼ�ѧ����ʽΪ___________________��
������������������Ӧ����������ʼ���дﵽƽ������¶ȡ�������䣬��Ҫ��ش��������⣺
(1)PCl5(g)![]() PCl3(g)��Cl2(g)
PCl3(g)��Cl2(g)
�ٳ���PCl5(g)��ƽ����___________�����ƶ�(�����Ӧ�����淴Ӧ��)���ﵽƽ���PCl5(g)��ת����___________(���������С�����䡱����ͬ)��
(2)2HI(g)![]() I2(g)��H2(g)
I2(g)��H2(g)
�ٳ���HI(g)��ƽ����___________�����ƶ����ﵽƽ���HI�ķֽ���___________��
(3)2NO2(g)![]() N2O4(g)
N2O4(g)
�ٳ���N2O4(g)��ƽ����___________�����ƶ����ﵽƽ���NO2(g)��ת����___________��
���𰸡���. (1)CH3OH(l)+![]() O2(g)=CO2(g)+2H2O(l) ��H=-704. 0kJ/mol
O2(g)=CO2(g)+2H2O(l) ��H=-704. 0kJ/mol
(2)N2(g)+2O2(g)=2NO2(g) ��H=+64.0kJ/mol
��. (1)����Ӧ ��С (2)����Ӧ ���� (3)�淴Ӧ ����
��������
�����������.(1)��25����101kPa�£�1g�״�(CH3OH)ȼ������CO2��Һ̬ˮʱ����22.0kJ��32g�״�ȼ�����ɶ�����̼��Һ̬ˮ�ų�����Ϊ704.0KJ�����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ��CH3OH(l)+![]() O2(g)=CO2(g)+2H2O(l)��H=-704.0kJmol-1��
O2(g)=CO2(g)+2H2O(l)��H=-704.0kJmol-1��
(2)������N2��O2��ȫ��Ӧ��ÿ����23��NO2��Ҫ����16.0kJ����������ÿ����92��NO2��Ҫ����64.0kJ���������Ȼ�ѧ����ʽΪ��N2(g)+2O2(g)=2NO2(g)��H=64.0kJmol-1��
����(1)���ڷ�ӦPCl5(g)![]() PCl3(g)��Cl2(g)�ں��º��������£��ٳ���PCl5(g)������Ӧ��Ũ�ȣ�ƽ��������Ӧ�����ƶ������ﵽƽ���PCl5(g)��ת���ʼ�С��
PCl3(g)��Cl2(g)�ں��º��������£��ٳ���PCl5(g)������Ӧ��Ũ�ȣ�ƽ��������Ӧ�����ƶ������ﵽƽ���PCl5(g)��ת���ʼ�С��
(2)���ڷ�Ӧ2HI(g)![]() I2(g)��H2(g)�ں��º��������£��ٳ���HI(g)��ƽ��������Ӧ�����ƶ����ﵽƽ���HI�ķֽ���������
I2(g)��H2(g)�ں��º��������£��ٳ���HI(g)��ƽ��������Ӧ�����ƶ����ﵽƽ���HI�ķֽ���������
(3)���ڷ�Ӧ2NO2(g)![]() N2O4(g)�ں��º��������£��ٳ���N2O4(g)��ƽ�����淴Ӧ�����ƶ����ﵽƽ���NO2(g)��ת����������
N2O4(g)�ں��º��������£��ٳ���N2O4(g)��ƽ�����淴Ӧ�����ƶ����ﵽƽ���NO2(g)��ת����������


 Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������Ϊ������Ҫ��������⣬���������������
A. CO2�������ӻᵼ������ЧӦ
B. Ϊ����ľ�ĵ�ʹ�ã�ʹ�þ�����ϩ������װ��
C. װ�����еļ�ȩ���������Ȼ������Ⱦ
D. ��Ȼ������ú��ȼ�Ͽɼ��ٻ�����Ⱦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ���ǣ� ��
A. ��H2O2�ֽ����1molO2��������ת�Ƶĵ�����ԼΪ4��6.02��1023
B. �����£�pH=3��CH3COOH��Һ��pH=11��NaOH��Һ�������ϣ���ҺpH��7
C. ����ˮբ����������������ӵ�����������������ֹ�丯ʴ
D. һ�������·�ӦN2+3H22NH3�ﵽƽ��ʱ��3v����H2��=2v����NH3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������¶��£���3�������Ϊ1.0L�ĺ����ܱ������з�Ӧ2H2(g)+CO(g)![]() CH3OH(g)�ﵽƽ�⡣����˵��������ǣ� ��
CH3OH(g)�ﵽƽ�⡣����˵��������ǣ� ��
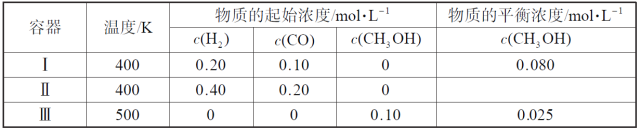
A���÷�Ӧ������Ӧ����
B���ﵽƽ��ʱ������I�з�Ӧ��ת���ʱ�����II�е�С
C���ﵽƽ��ʱ������II��c(H2)��������III�е�����
D���ﵽƽ��ʱ������III�е�����Ӧ���ʱ�����I�еĴ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����pH=5��H2SO4����Һϡ��1000������Һ��SO42-����Ũ����H+����Ũ�ȵı�ֵԼΪ
A. 1��10 B. 1��1 C. 1��2 D. 1��20
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ھ��������˵����ȷ���ǡ�(����)
A. ԭ�Ӿ�����ֻ���й��ۼ�
B. �κξ�����,�����������Ӿ�һ����������
C. ԭ�Ӿ�����۵�һ���Ƚ�������ĸ�
D. ���Ӿ����к������Ӽ��������ܺ��й��ۼ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��1���pH=2��5��������10����ijһԪǿ����Һǡ����ȫ��Ӧ����ü���Һ��pH������ ��
A��9��0 B��9��5 C��10��5 D��11��0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Cl2����ijЩ�����л���ʱ�����������HC1�����÷�ӦA����ʵ���ȵ�ѭ�����á�
��ӦA��4HCl��O22Cl2��2H2O
��1����֪:������ӦA�У� 4mol HCl���������ų�115.6kJ��������
����![]()
����ӦA���Ȼ�ѧ����ʽ��________________________��
���Ͽ�1 mol H��O ����Ͽ� 1 mol H��Cl �������������ԼΪ__________kJ��H2O��H��0 ����HCl��H��Cl��������ǿ������������_______________��
��2���ϳɰ�������ͨ���ⶨ��Ӧǰ����������ܶ���ȷ������ת���ʡ�ij������úϳ�����N2��H2���������ܶ�Ϊ0.5536g/L����״�������Ӻϳ����г����Ļ����������ͬ�������ܶ�Ϊ0.693g/L����״�������úϳɰ���N2��ת����Ϊ___________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com