
����Ŀ��Ԫ�ؼ��仯�����Ӧ�ù㷺���ش��������⣺
��1�������к���KIO3��KIO3�е�Ԫ�صĻ��ϼ���____________��
��2��84����Һ����Ч�ɷ���____________������NaCl������NaClO������
��3��������Fe2O3��CO��Ӧ�ǹ�ҵ��������ԭ������ԭ���Ļ�ѧ��Ӧ����ʽΪ____________����Ӧ��Fe2O3��________��������������������ԭ��������
��4��ʵ������NaOH��Һ���պ�SO2��β�����÷�Ӧ�����ӷ���ʽΪ____________��SO2����____________��������������������������������������SO2��ʹƷ����Һ��ɫ��˵��SO2���е�������____________��
���𰸡�+5�� NaClO Fe2O3+3CO![]() 3CO2+2Fe ������ SO2+2OH-===SO32-+H2O ���������� Ư����
3CO2+2Fe ������ SO2+2OH-===SO32-+H2O ���������� Ư����
��������
(1)���ݸ�Ԫ�صij������ϼ���⣬K��+1�ۣ�O��-2�ۣ�����I��+5��(2) 84����Һ�����ô������ε�ǿ������ɱ��������(3)���������ص��ǵõ��ӣ����ϼ۽��͡�(4)��������������Ӧ�����κ�ˮ��
(1) K��+1�ۣ�O��-2�ۣ�����I��+5��
(2) 84����Һ�����ô������ε�ǿ������ɱ������������Ч�ɷ���NaClO��
(3)��ҵ������������CO��ԭFe2O3�õ������ʣ����Ը÷�Ӧ��Fe2O3+3CO![]() 3CO2+2Fe������Fe2O3���ϼ۽��ͣ�����ԭ������������
3CO2+2Fe������Fe2O3���ϼ۽��ͣ�����ԭ������������
(4) SO2��Ӧ�����κ�ˮ����������������Ķ��塣SO2����Ư���ԣ���ʹƷ����Һ��ɫ��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ��������Ի����л����ɵ���S��
�����Ʊ�Na2S2O3��5H2O��Ӧԭ����Na2SO3(aq)��S(s)![]() Na2S2O3(aq)
Na2S2O3(aq)
ʵ�鲽�裺
�ٳ�ȡ15gNa2SO3����Բ����ƿ�У��ټ���80 mL����ˮ����ȡ5g��ϸ����ۣ���3mL�Ҵ���ʪ������������Һ�С�
�ڰ�װʵ��װ��(��ͼ��ʾ�����ּг�װ����ȥ)��ˮԡ���ȣ���60min���ش��������⣺

��1������ڷ�Ӧǰ���Ҵ���ʪ��Ŀ����_____��
��2������a��������______��
��3����Ʒ�г�����δ��Ӧ��Na2SO3�⣬����ܴ��ڵ���������____�������Ƿ���ڸ����ʵķ�����______��
����Na2S2O3��5H2O��֪��Na2S2O3��5H2O��40��45���ۻ���48��ֽ⣻Na2S2O3 ������ˮ���������Ҵ�����ˮ���й����ʵ��ܽ��������ͼ��ʾ��

����Na2S2O3��5H2O�����������ͼ��ʾ��

�ش��������⣺
��1��Ϊ���ٲ�Ʒ����ʧ��������Ϊ___���������dz���ϴ�Ӹ������ϴ�Ӳ�������___�����Լ�����ϴ�Ӽ���
��2������Ũ����Һֱ����Һ����ɫ����Ϊֹ������ʱΪʲôҪ�����¶Ȳ��˹���_____��
III���ⶨ��Ʒ����
ȷ��ȡWg��Ʒ������������ˮ�ܽ⣬�Ե�����ָʾ������0.1000mol��L��1��ı���Һ�ζ�����Ӧԭ��Ϊ2S2O32-��I2=S4O62-��2I��
��1���ζ����յ�ʱ����Һ��ɫ�ı仯_____��
��2���ζ���ʼ���յ��Һ��λ����ͼ�������ĵ�ı���Һ���Ϊ___mL��

��Ʒ�Ĵ���Ϊ(��Na2S2O3��5H2O��Է�������ΪM)___��
IV��Na2S2O3��Ӧ��
Na2S2O3��ԭ�Խ�ǿ������Һ���ױ�Cl2������SO42-�����������ȼ����÷�Ӧ�����ӷ���ʽΪ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Խ�����Ʒ���и㸯ʴ���������ӳ���ʹ��������
��1������Ϊ������洦����һ�ַ�����

����ϴ��Ŀ���dz�ȥ����������Ȼ����Ĥ����ϴ��ʱ��������ð����ԭ���ǣ�________�������ӷ���ʽ��ʾ����Ϊ����ϴ��Һ�е����Գ�����ʽ���գ�������Һ�м��������Լ��е�__________��
a.NH3b.CO2c.NaOH d.HNO3
��������Ϊ��������H2SO4��Һ�е�⣬��������γ�����Ĥ�������缫��ӦʽΪ��_____��
ȡ�����ϵ��Һ������NaHCO3��Һ��������ݺͰ�ɫ����������������ԭ����_________��
(2)��ͭ�ɷ�ֹ����Ʒ��ʴ�����ʱ��ͭ������ʯī��������ԭ���� ��
��3��������ͼװ�ã�����ģ�����ĵ绯ѧ������

��XΪ̼����Ϊ�������ĸ�ʴ������KӦ������ ����
��XΪп������K����M�����õ绯ѧ��������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ӧ�ù㷺�Ľ�������������(��Ҫ�ɷ�ΪAl2O3����SiO2��Fe2O3������)Ϊԭ���Ʊ�����һ�ֹ����������£�

ע��SiO2����������ʱת��Ϊ�������Ƴ�����
��1����������ʱ����ƫ�����Ƶ����ӷ���ʽΪ_____________________��
��2������������������Һ�м���NaHCO3��Һ����Һ��pH_________ (��������������������������С��)��
��3����������ǵ������Al2O3������������������ʯī�����ģ�ԭ����___________��
��4����������ǵ��Na2CO3��Һ��ԭ����ͼ��ʾ�������ĵ缫��ӦʽΪ_____________________����������������A�Ļ�ѧʽΪ____________��
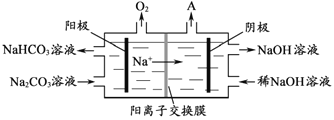
��5��������1000��ʱ����N2��Ӧ�Ʊ�AlN������������������NH4Cl���岢��ֻ�ϣ�������AlN���Ʊ�������Ҫԭ����_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼΪCaF2��H3BO3(��״�ṹ�����ڵ�H3BO3����ͨ��������)������ͭ���־���Ľṹʾ��ͼ����ش��������⣺


ͼ��ͭ������ͭԭ�Ӷѻ�ģ��
(1)ͼ����ʾ��CaF2��������Ca2������ҵȾ����F����Ϊ________________��ͼ����δ��ŵ�ͭԭ���γɾ������Χ����ڵ�ͭԭ����Ϊ__________________________________��
(2)ͼ����ʾ�����ʽṹ�������ܲ��Ѵ�8���ӽṹ��ԭ����________��H3BO3������Bԭ�Ӹ����뼫�Լ�������Ϊ____________��
(3)����ͭ���кܺõ���չ�ԡ������ԡ������ԣ��Դ�������Ľ�������________���ۡ�
(4)���־������۵���͵���________(�ѧʽ)���侧�������ۻ�ʱ���˷�����֮��������Ϊ____________________________________________________________��
(5)��֪�������������Ca2���˼����Ϊa��10��8cm�����CaF2����ľ���ʾ��ͼ��CaF2������ܶ�Ϊ_______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������йص������Һ��˵����ȷ����![]() ����
����![]()
A. ��![]() ��Һ�м�������ˮ����Һ��
��Һ�м�������ˮ����Һ��![]() ��С
��С
B. ��![]() ��Һ��
��Һ��![]() ������
������![]() ����Һ��
����Һ��![]() ����
����
C. �������м��백ˮ�����ԣ���Һ��![]()
D. ��AgCl��AgBr�ı�����Һ�м�������![]() ����Һ��
����Һ��![]() ����
����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ԫ�� R��X��T��Z��Q ��Ԫ�����ڱ��е����λ����ͼ��ʾ������ R ���⻯���ˮ��Һ����������ʴ�������������ж���ȷ����( )

A.��̬�⻯������ԣ�R>T>Q
B.��̬�⻯��Ļ�ԭ�ԣ�X>T
C.R �� Q �ĵ�������� 16
D.R �ĵ���ͨ�� T ��������Һ�����û���T �ĵ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ǧ��ұ�����¹������£�
������������Ǧ��PbS�����и�ѡ�������գ�2PbS��3O2![]() 2PbO��2SO2��
2PbO��2SO2��
���ƴ�Ǧ��PbO��C![]() Pb��CO��PbO��CO
Pb��CO��PbO��CO![]() Pb��CO2��
Pb��CO2��
����˵����ȷ���ǣ� ��
A. ��ѡ��������Ǧ��Ĺ������ڻ�ѧ�仯
B. ��Ǧ���շ�Ӧ�У�PbS�ǻ�ԭ����������ԭ��Ӧ
C. �������У���ȡ1molPbO��ת��2mol����
D. ��1molPbSұ����Pb������������Ҫ6g̼
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ǹ�[(CH3COO)2Cr��H2O��Ϊש��ɫ���壬��������ˮ���������ᣬ����������������������ռ���һ���Ʊ����������ڷ����ϵ�����ý���п����ԭ���������۸���ԭΪ���۸������۸������������Һ���ü����Ƶô����Ǹ���ʵ��װ����ͼ��ʾ���ش��������⣺

��1��ʵ������������ˮ���辭��к�Ѹ����ȴ��Ŀ����_________������a��������_______��
��2��������п�����Ȼ�����������c�У�������������ˮ����ͼ���Ӻ�װ�ã���K1��K2���ر�K3��
��c����Һ����ɫ��Ϊ����ɫ���÷�Ӧ�����ӷ���ʽΪ_________��
��ͬʱc��������������������������_____________��
��3����K3���ر�K1��K2��c������ɫ��Һ����d����ԭ����________��d������ש��ɫ������Ϊʹ����������������룬����õIJ�����___________��_________��ϴ�ӡ����
��4��ָ��װ��d���ܴ��ڵ�ȱ��______________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com