
ijУ��ѧʵ��С��Ϊ��֤��ͭ��ϡ���ᷴӦ����һ������������ͼ��ʾװ�ý���ʵ��(����װ�úͼг�װ�þ�����ȥ��װ�����������ã�F�����ڹ��������˫��������)��
ʵ�����������
| ʵ����� | ���� |
| ��.��Bװ�����ƣ�ʹ̼�����ϡ����Ӵ� | �������� |
| ��.��Cװ���в�����ɫ����ʱ�����̽�Bװ������ | |
| ��.��Aװ����ͭ˿����ϡ�����У���Aװ������ | Aװ���в�����ɫ���� Eװ���п�ʼʱ����dz����ɫ���� |
| ��.��Fװ����Eװ���й������ | Eװ����������ɫ���� |
| ��.һ��ʱ��� | Cװ���а�ɫ�����ܽ� |
(1)CO2����̼�����ϡ���ᷴӦ�����Ķ�����̼����װ���ڵĿ�����ƽ��ѹǿ������NO��NO2��CO2β������ֹ��Ⱦ����
(2)CaCO3����ֹ����ϡ������࣬�Ӷ�Ӱ��ͭ��ϡ����ķ�Ӧ
(3)3Cu��8HNO3(ϡ)=3Cu(NO3)2��2NO����4H2O
(4)CO2���ܶȱȿ����Ĵ�CO2�ӳ��ܽ���Eװ�ã���û�а�Eװ���еĿ����ž�����ʹ����NO����δ�ž��Ŀ�����Ӧ������������ɫNO2������Fװ�ù������ʱ��Eװ�����и���NO2���ɣ����Ժ���ɫ����
(5)3NO2��H2O=2HNO3��NO(��4NO��3O2��2H2O=4HNO3)��CaCO3��2HNO3=Ca(NO3)2��CO2����H2O
����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��20 mL NO2��NH3�Ļ�����壬��һ�������³�ַ�Ӧ����ѧ����ʽΪ�� 6NO2+8NH3=7N2+12H2O����֪�μӷ�Ӧ��NO2�Ȳμӷ�Ӧ��NH3��2 mL���������������ͬ״���²ⶨ������ԭ���������NO2��NH3�����ʵ���֮���ǣ� ��
��3:2 ��2:3 ��3:7 ��3:4
| A���٢� | B���ڢ� | C���٢� | D���ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��11�� ijУ��ѧ��ȤС���ͬѧ�������ε����ȷֽ����̽�����������������װ�÷ֱ������Ca��NO3��2��Cu(NO3)2��AgNO3���ֹ��塣�����ȼ��г�װ��δ������
��1����ͬѧ���ȵ���Ca��NO3��2�����ȹ��̷��֣�װ�â��в��� ���ݣ�����ʯ����Һ��ѹ��װ�â��У��ô����ǵ�ľ��������е����壬ľ����ȼ������װ�â���ʣ��Ĺ����֪��ʣ������к���NԪ�أ����ԣ�3�ۡ���д��Ca��NO3��2���ȷֽ�����ɲ���Ļ�ѧʽ�� �� ��
��2����ͬѧ���ȵ���Cu(NO3)2�����ȹ��̷��֣�װ�â���Ҳ�����ݲ��������������Ĺ�������ʧ��ʯ����Һ��Ϊ��ɫ��Һ�弸������ѹ��װ�â��С�װ�â��еĹ�����Ϊ��ɫ����д��Cu(NO3)2���ȷֽ�Ļ�ѧ����ʽ�� ��
��3����ͬѧ���ȵ���AgNO3�����ȹ��̷��֣�װ�â���Ҳ�����ݲ��������������Ĺ��������ݲ�����ʧ��ʣ�������Ҳ��ʹ�����ǵ�ľ����ȼ��ʯ����ҺҲ��Ϊ��ɫ��������Һ�屻ѹ��װ�â��С�װ�â��еĹ�����Ϊ��ɫ����ͬѧ�ݴ�д����AgNO3���ȷֽ���ܵ����ֻ�ѧ����ʽ��
����4AgNO3  2Ag2O��4NO2����O2�� ����2AgNO3
2Ag2O��4NO2����O2�� ����2AgNO3 2Ag��2NO2����O2����
2Ag��2NO2����O2����
������ȷ���� ����˵�����ɣ� ��
�������һ����ʵ��֤����Ľ�������ȷ�ģ� ��
��4��������3��ʵ��Ľ���������Ʋ����������ȷֽ�Ĺ��ɣ� ��
��5����������ͬѧ����������ag��������������ȫ�ֽ��ȡ��Ͳ���Ϊbml�����������ķֽ��ʣ�____________�����������������ϵ���ɲ��û���С����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ǵؿ��к�����Ϊ�ḻ�ķǽ���Ԫ��,��Ҫ��������ˮ����������Ca3(PO4)2����ʽ���ڡ����ĵ��ʺͻ������ڹ�ũҵ������������Ҫ��Ӧ�á�
��1������(P4)����Ca3(PO4)2����̿��SiO2��һ�������·�Ӧ��á�����Ȼ�ѧ����ʽ����:
��2Ca3(PO4)2(s)+10C(s) 6CaO(s)+P4(s)+10CO(g����H1="+3" 359��26 kJ��mol-1
6CaO(s)+P4(s)+10CO(g����H1="+3" 359��26 kJ��mol-1
��CaO(s)+SiO2(s) CaSiO3(s�� ��H2=-89��61 kJ��mol-1
CaSiO3(s�� ��H2=-89��61 kJ��mol-1
2Ca3(PO4)2(s)+6SiO2(s)+10C(s) 6CaSiO3(s)+P4(s)+10CO(g����H3��H3= kJ��mol-1��
6CaSiO3(s)+P4(s)+10CO(g����H3��H3= kJ��mol-1��
��2�������ж������CuSO4��Һ�ⶾ,�ⶾԭ���������л�ѧ����ʽ��ʾ: 11P4+60CuSO4 +96H2O 20Cu3P +24H3PO4+60H2SO4 6 mol CuSO4�������������ʵ����� ��
20Cu3P +24H3PO4+60H2SO4 6 mol CuSO4�������������ʵ����� ��
��3������Ҫ������NaH2PO4��Na2HPO4��Na3PO4��ͨ��H3PO4��NaOH��Һ��Ӧ���,�������ֵķֲ�����(ƽ��ʱij���ֵ�Ũ��ռ������Ũ��֮�͵ķ���)��pH�Ĺ�ϵ��ͼ��ʾ��
��Ϊ��þ����ܴ���NaH2PO4,pHӦ�������� pH=8ʱ,��Һ����Ҫ��������Ũ�ȴ�С��ϵΪ ��
��Na2HPO4��Һ�Լ���,������Һ�м���������CaCl2��Һ,��Һ��������,��ԭ���� (д���ӷ���ʽ)��
��4���Ļ�������������( )�뼾���Ĵ�(
)�뼾���Ĵ�( )�����ʵ���֮��2��1��Ӧʱ,�ɻ��һ��������ȼ���м���X,���ͷų�һ���������塣�����Ĵ���X�ĺ˴Ź�����������ͼ��ʾ��
)�����ʵ���֮��2��1��Ӧʱ,�ɻ��һ��������ȼ���м���X,���ͷų�һ���������塣�����Ĵ���X�ĺ˴Ź�����������ͼ��ʾ��
������������ (�ѧʽ)
��X�Ľṹ��ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼÿһ�����е���ĸ����һ�ַ�Ӧ������������J�Ǻ�����Ԫ��A�İ�ɫ��״������IΪNaCl��Һ��D�ǵ���ɫ���嵥�ʡ�����д���пհס�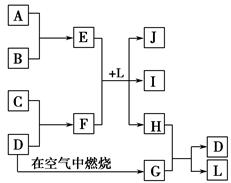
(1)��ͼ���������������ڷǵ���ʵ����ʵĻ�ѧʽ�� ��
(2)�õ���ʽ��ʾ��H���γɹ��� ��
(3)��E��ˮ��Һ���ɲ����յõ��Ĺ������ʵĻ�ѧʽΪ ��
(4)F��ˮ��Һ�и�����Ũ���ɴ�С��˳��Ϊ ��
(5)F��ˮ��Һ�Լ��Ե�ԭ�� (�����ӷ���ʽ��ʾ)��
(6)E��F��L�з�Ӧ�����ӷ���ʽΪ ��
(7)H��G֮�䷴Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����Ǽ�����Ҫ�Ļ���ԭ�ϣ���ҵ�Ͻ�������NO2���ܱ���������ˮ��η���ѭ�������Ʊ����ᡣ
(1)��ҵ����ˮ����NO2����HNO3�����ɵ����徭������������յ�ѭ���������ת��Ϊ����(�ٶ�����������������ʧ)����д��������Ӧ�Ļ�ѧ����ʽ��
��
(2)Ϊ��֤��NOҲ������������ˮ��ͬ��Ӧ����HNO3��ijѧ���������ͼ��ʾװ��(�йؼг�װ������ȥ)��
�ټ��װ�����������ú�Ϊ�۲쵽NO�������ɣ���K1���ر�K2��Ӧ��U�ιܵij��ܿ�ע��ϡ������ ��Ѹ�ٹر�K1���۲쵽U�ι��ڵ������� ��
��װ�â��з�����Ӧ�Ļ�ѧ����ʽΪ ��
��պNaOH��Һ�����ŵ������� ��
�ܴ�K2����װ�â��г��������е��������ɫ��K3����Ӧһ��ʱ����������в�δ����Һ�塣��Ƽ������鳤�������е������Ƿ�NO ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ�Ͽ���ͭм��Ũ����Ϊԭ����ȡ����ͭ����ʵ�������У��Ȱ�ͭм�ڿ��������գ��ٸ��õ����ˮϡ�͵�Ũ���ᷴӦ����ȡ����ͭ����ش��������⣺
(1)��������ͭмֱ�������ᷴӦ����ȡ����ͭ��ԭ���� ��
(2)Ũ�����õ����ˮϡ�͵�Ŀ���� ��
(3)Ҫ�õ�����ͭ���壬Ӧѡ�� ��
(4)��Ӧ��������ֳ� �ԡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ֻ�������һ���������õIJ����������Կ˷������ʴ������ȱ�㡣���ӽ������Dz������иֻ���һ����Ҫ����������ͨ���ƸƲ�����̼��ص�ǿ����(475 ��)�н���3.5Сʱ���Ƶá���ͼ�����ӽ�������ʾ��ͼ��
(1)��ͨ��������ʯӢɰ��__________��__________�������ڶ��ɡ�
(2)��ͨ��������������ƺ�dz��������飬����������Ƶ�����֮һ������ᣬд��������벣����Ӧ�Ļ�ѧ����ʽ________________��
(3)�������иֻ������У����ӽ�����__________(������仯����ѧ�仯��)��
(4)�ڲ������иֻ�����ʱ���ܷ���̼����ش���̼��أ�__________(��ܡ����ܡ�)��ԭ����______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������һ����Ҫ�������ܲ���,ij�о���ѧϰС����Ƶ����ú��ߵ�ʯӢɰ(���Ȼ��ơ�������������)�Ʊ������Ƶ���������: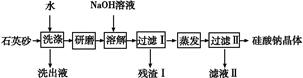
(1)ʯӢɰ��ˮϴ�ӵ�Ŀ���� ��
��ĥ�����NaOH��Һ�ܽ�����ӷ���ʽ�� ����
(2)ʵ���ҽ�����������Ҫ�õ�������������̨(����Ȧ)����������������������
(3)������ijɷ�����������(�ѧʽ)������������������,�ټ���NaOH��Һ�õ�����,���ó������뵽NaClO��NaOH�����Һ�п��Ƶ�һ�����ʾ�ˮ��,��ɷ�Ӧ�����ӷ���ʽ:���� ������+��ClO-+��OH- ���� ������+��Cl-+��H2O
���� ������+��Cl-+��H2O
(4)�����������ƵõĹ����ƾ���ɱ�ʾΪNa2O��nSiO2,��ʯӢɰ������Ϊ10.0 g,���к�SiO2����������Ϊ90%,���յõ������ƾ���15.2 g,��n=����������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com