
 __________________________________
__________________________________ H2�� + 2OH�� + Cl2�� ��2�֣�
H2�� + 2OH�� + Cl2�� ��2�֣� H2�� + 2OH�� + Cl2��
H2�� + 2OH�� + Cl2�� 

 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д� ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Na2SO4 | B��NaNO3 | C��KOH | D��Cu SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������ʵ��ʱ�������������� |
| B����ⴿ��ˮ������ˮʱ������ͬ����Ϊ����ˮ������ˮ�����������ҺŨ�Ȳ�ͬ |
| C�������缫�����ĵ缫��ӦΪAl��3e��===Al3�� |
| D���������ˮʱ���֡����ɫ�����ɫ����״�����Ϊ������������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
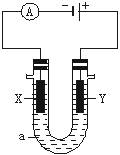
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | NaOH���������� | �����������ʵ�����/g | �����������ʵ�����/g |
| A | 0.062��6.2%�� | 19 | 152 |
| B | 0.062��6.2%�� | 152 | 19 |
| C | 0.042��4.2%�� | 1.2 | 9.4 |
| D | 0.042��4.2%�� | 9.4 | 1.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | �� | �� | �� | �� |
| A | Pt | Pt | CuCl2 | HCl���� |
| B | C | Cu | CuSO4 | CuO���� |
| C | Pt | C | H2SO4 | H2SO4������ |
| D | Fe | Fe | NaCl | NaOH���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�� 6.72L | B�� 8.96L | C��11.2L | D��5.6L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


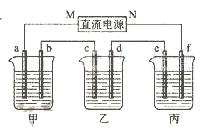
 ��1����ͨ��Դ������һ��ʱ���ñ���K2SO4Ũ��Ϊ10.47%������c�缫�������ӡ�
��1����ͨ��Դ������һ��ʱ���ñ���K2SO4Ũ��Ϊ10.47%������c�缫�������ӡ� �ٵ�Դ��N��Ϊ ����
�ٵ�Դ��N��Ϊ ���� �ڵ缫b�Ϸ����ĵ缫��ӦΪ ��
�ڵ缫b�Ϸ����ĵ缫��ӦΪ �� �۵缫b�����ɵ������ڱ�״���µ������
�۵缫b�����ɵ������ڱ�״���µ������
 �ܵ缫c�������仯�� g��
�ܵ缫c�������仯�� g�� ��2�������������ͭȫ����������ʱ����ܷ�������У�Ϊʲô��
��2�������������ͭȫ����������ʱ����ܷ�������У�Ϊʲô��  ��
�� �ݵ��ǰ�����Һ��pH��α仯����������С�䣩
�ݵ��ǰ�����Һ��pH��α仯����������С�䣩 ����Һ ��
����Һ �� ����Һ�ߣߣߣߣߣߣߣ�
����Һ�ߣߣߣߣߣߣߣ� ����Һ�ߣߣߣߣߣߣߣ�
����Һ�ߣߣߣߣߣߣߣ��鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com