
��1��ijһ��Ӧ��ϵ���з�Ӧ��������ﹲ5�����ʣ�S��H2S ��HNO3��NO ��H2O����÷�Ӧ�л�ԭ������_____������Ӧ������ת����0.3mol���ӣ������������������_____g��
��2����a mol Cl2ͨ�뺬b mol FeBr2����Һ�У���0< a / b��1/2 ʱ����Ӧ�����ӷ���ʽΪ��
2Fe2+ + Cl2 = 2Fe3+ + 2Cl����д����2�����ܷ��������ӷ���ʽ��
�ٵ� a / b =1ʱ ��
�ڵ�a / b��3/2ʱ ��
��3���۲����·�Ӧ���ܽ���ɣ�Ȼ������������⣺
��Al(OH)3��H2O  Al(OH)4��+ H+ ���� Cl2+2OH��= Cl��+ ClO��+H2O
Al(OH)4��+ H+ ���� Cl2+2OH��= Cl��+ ClO��+H2O
������֪B(OH)3��һԪ���ᣬ��д������뷽��ʽ�� ��
��������(CN)2����±�أ���д����������������Һ��Ӧ�����ӷ���ʽ ��
��1��NO(2��) �� 4.8g (2��)
��2�� ��2Cl2 + 2Fe2+ + 2Br�D = 2Fe3+ + Br2 + 4Cl�D (3��)
��3Cl2 + 2Fe2+ + 4Br�D = 2Fe3+ + 2Br2 + 6Cl�D (3��)
��3������B(OH)3��H2O  B(OH)4�D + H+ (2��)
B(OH)4�D + H+ (2��)
����(CN)2 +2OH�D �� CN�D + CNO�D +H2O (2��)
���������������1������������Դ����������ڸ÷�Ӧ�����������������ڷ�Ӧ�еõ���N���ϼ۽��ͱ���ԭ����ԭ������NO�������ڷ�Ӧ��ʧ���ӱ�����S���ϼ۽����������ʣ����Ը÷�Ӧ�ķ���ʽΪ��3H2S+2HNO3=3S+2NO+4H2O��������3molSʱ����Ӧת�Ƶ��ӵ����ʵ�����6mol��������Ӧ������ת����0.3mol���ӣ���Ӧ���ɵ�S��0.15mol,��������Ϊ32g/mol��0.15mol=4.8g��
��2��FeBr2����Һ�У���ԭ��Fe2+ >Br�D ������ͨ��������Fe2+ �ȱ�������������Ӧ�����ͨ������Br�D �����������Ե�0< a / b��1/2ʱ��ֻ��Fe2+ �����������Է�����Ӧ2Fe2+ + Cl2 = 2Fe3+ + 2Cl�� ���� a / b =1ʱ��Fe2+ ȫ����������Br�D ��Fe2+ ��2����ֻ��1�뱻���������Է����ķ�Ӧ����ʽΪ2Cl2 + 2Fe2+ + 2Br�D = 2Fe3+ + Br2 + 4Cl�D ����a / b��3/2ʱ��Fe2+ ��Br�Dȫ�������������Է�ӦΪ3Cl2 + 2Fe2+ + 4Br�D = 2Fe3+ + 2Br2 + 6Cl�D��
��3����ΪB(OH)3��һԪ���ᣬ������������������ƫ���ᣩ�ĵ��룺Al(OH)3��H2O  Al(OH)4��+ H+ ������B(OH)3 Ӧ������ˮ���ã�Ҳ����������ӣ�B(OH)3��H2O
Al(OH)4��+ H+ ������B(OH)3 Ӧ������ˮ���ã�Ҳ����������ӣ�B(OH)3��H2O  B(OH)4�D + H+ ��������(CN)2����±�أ���������±�ص������ƣ�������������������Һ��ӦҲӦ�����������Σ���CN����һ��±��ԭ�ӣ����������ΪCN-��CNO- ,�������ӷ���ʽΪ��(CN)2 +2OH�D �� CN�D + CNO�D +H2O��
B(OH)4�D + H+ ��������(CN)2����±�أ���������±�ص������ƣ�������������������Һ��ӦҲӦ�����������Σ���CN����һ��±��ԭ�ӣ����������ΪCN-��CNO- ,�������ӷ���ʽΪ��(CN)2 +2OH�D �� CN�D + CNO�D +H2O��
���㣺���⿼�����������ԭ��Ӧ����ʽ��ƽ������������ʵĵ��롣


 �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д� ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ƣ�NaClO2��������ˮ��������ɰ�ǡ���֬��Ư����ɱ�����������ù������ⷨ�����������ƵĹ�������ͼ��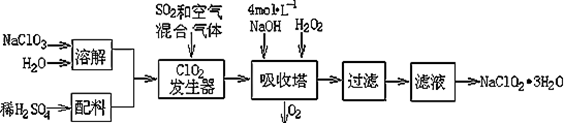
��֪����NaClO2���ܽ�����¶����߶������ʵ������¿ɽᾧ����NaClO2��3H2O ;
��Ksp(FeS)��6��3��10-18 �� Ksp(CuS)��6��3��10-36 ��Ksp(PbS)��2��4��10-28
��1���������ڷ�����Ӧ�����ӷ���ʽΪ ���ù��������е�NaClO3��ClO2��NaClO2����ǿ�����������Ƕ��ܺ�Ũ���ᷴӦ��ȡCl2�����ö������Ⱥ�Ũ������ȡCl2��������5 mol Cl2ʱ��ͨ����ԭ��Ӧ�Ƶ�����������Ϊ g��
��2������Һ�еõ�NaClO2��3H2O������������������ ����д��ţ���
a����b���գ�c���ˣ�d��ȴ�ᾧ��e����
��3��ӡȾ��ҵ�����������ƣ�NaClO2��Ư��֯�Ư��֯��ʱ���������õ���HClO2��
�±��� 25��ʱHClO2�����ֳ�������ĵ���ƽ�ⳣ����
| ���� | HClO2 | HF | HCN | H2S |
| Ka/mol?L-1 | 1��10-2 | 6��3��10-4 | 4��9��10-10 | K1��9��1��10?8 K2��1��1��10?12 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Χ�������������ʣ���������A12O3����ϡ���ᣬ��H2SO4����Ba(OH)2���壬�ް�ˮ����A12(SO4)3������Ҫ��ش��������⡣
��1��������ǿ�ᷴӦ��������ǿ�Ӧ���� ������ţ���
��2�����ڵ���ʵ��� ������ţ���
��3��д����������ȡAl(OH)3�����ӷ���ʽ ��
��4�������ʷ�����Ӧ�����ӷ���ʽΪH++OH��=H2O����д���÷�Ӧ�Ļ�ѧ����ʽ ��
��5��34.2g������ˮ���500mL��Һ����Һ��SO42�������ʵ���Ũ��Ϊ ��
��6������۷�����Ӧ�Ļ�ѧ����ʽΪA1+4HNO3=A1(NO3)3+NO��+2H2O���÷�Ӧ�л�ԭ���������������ʵ���֮���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�����Һ�п��ܴ������е��������±���ʾ��
| ������ | H����K����Al3����NH4+��Mg2�� |
| ������ | Cl����Br����OH����CO32-��AlO2- |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����һ������ˮ��Һ��ֻ���ܺ������������е������֣�K+��NH4+��Cl-��Ca2+��Ba2+��CO32-��SO42-. ��ȡ���ݸ�100mL��Һ��������ʵ�飺
��һ�ݼ���AgNO3��Һ�г���������
�ڶ��ݼ�������NaOH��Һ���Ⱥ��ռ���0.08mol���壻
�����ݼ�������BaCl2 ��Һ�õ��������12.54g��������������ϴ�ӡ������������Ϊ4.66g��
��������ʵ�飬�ش��������⣺
��1���ɵ�һ�ݽ��е�ʵ���ƶϸû�����Ƿ�һ������Cl- �� ��ԭ���� .
��2���ɵڶ��ݽ��е�ʵ���֪�������Ӧ���� ���ӣ������ʵ���Ũ��Ϊ .
��3���ɵ����ݽ��е�ʵ���֪12.54g �����ijɷ�Ϊ ��������γɸó�����ԭ������и����ӵ����ʵ�������Ҫ�������̣�
��4���ۺ�����ʵ�飬����Ϊ���½�����ȷ����
| A���û������һ������K+��NH4+��CO32-��SO42-�����ܺ���Cl-����n(K+)��0.04mol |
| B���û������һ������NH4+��CO32-��SO42-�����ܺ���Ca2+�� K+��Cl- |
| C���û������һ������NH4+��CO32-��SO42-�����ܺ���K+��Cl- |
| D���û������һ������NH4+��SO42-�����ܺ���Ca2+��K+��Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������ʣ����������ƹ��� ��ͭ˿ ������ �������Ȼ��� �ݶ�����̼���� �ް�ˮ �����Ǿ��塣���������գ�
(1)����״̬�¿ɵ������________�� (2)���ڵ���ʵ���________��
(3)���ڷǵ���ʵ���________�� (4)����״̬�µĵ���ʲ��ܵ������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Na��Ũ��Ϊ0��5mol��L��ij������Һ�У������ܺ����±��е����������ӣ�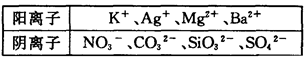
ȡ����Һ100mL��������ʵ�飨��������ڱ�״���²ⶨ����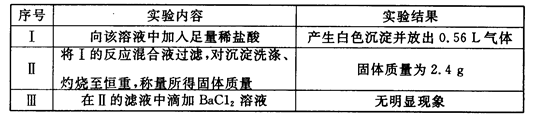
�Իش��������⣺
��1��ʵ�����ȷ��һ�������ڵ���������____________��
��2��ͨ��ʵ���ͱ�Ҫ���㣬��д�±��������ӵ�Ũ�ȣ��ܼ�����ģ���д��������һ�������ڵ������0��������ȷ���Ƿ���ڵ������?����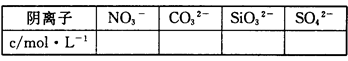
��3���ж�K���Ƿ���ڣ�������������СŨ�ȣ���������˵������_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
I����1����Ba(OH)2��Һ�м���ϡ���ᣬ������������⣺
��1��д����Ӧ�����ӷ���ʽ��________________________________��
��2��������������£����ӷ���ʽ�루1����ͬ����_________________��
A����NaHSO4��Һ����μ���Ba(0H)2��Һ��������
B����NaHSO4��Һ����μ���Ba(0H)2��Һ��SO42��ǡ����ȫ����
C����NaHSO4��Һ����μ���Ba(0H)2��Һ������
��ʵ���ҿ���������غ�Ũ���ᷴӦ��ȡ��������Ӧʽ���£�KClO3��6HCl(Ũ)��KCl��3Cl2����3H2O
��1����˫���ŷ���ʾ������Ӧ�е���ת�Ƶķ������Ŀ��
��2����Ӧ�з���������Ӧ��������____________���ѧʽ��������ԭ��Ԫ����____________________����Ԫ�����ƣ���
��3���������뻹ԭ�������ʵ���֮��Ϊ____________________��
��4������Ӧ�б�����������Ϊ1mol�������ɵ��������Ϊ_______________����״���£���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ȣ�ClO2����һ����ˮ�����ȷ����й㷺Ӧ�õĸ�Ч��ȫ��������������Cl2��Ȳ��������������DZ��Σ�����л��ȴ���Ʊ�ClO2���������ַ�����
����һ��2 NaClO3+4 HCl��2 ClO2��+Cl2��+2 NaCl+2 H2O
��������2 NaClO3+H2O2+H2SO4��2 ClO2��+O2��+2 Na2SO4+2 H2O
��1������һ�����ӷ���ʽΪ .
��2���������б������������� ������Ӧ����0.1mol����ת�ƣ��������ClO2�����ڱ�״���µ����Ϊ L.
��3���÷������Ʊ���ClO2���ʺ���������ˮ������������Ҫԭ���� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com