
����Ŀ��ij��Һ�п��ܺ�������6�������е�ij���֣�Cl��![]() ��
��![]() ��
��![]() ��K+��Na+��Ϊȷ����Һ��ɽ�������ʵ�飺
��K+��Na+��Ϊȷ����Һ��ɽ�������ʵ�飺
��200 mL������Һ����������BaCl2��Һ����Ӧ�������ˡ�ϴ�ӡ�����ó���4.30 g��������м�����������ᣬ��2.33 g�������ܡ�
����������Һ�м���������NaOH��Һ�����ȣ������ܴ�ʹʪ���ɫʯ����ֽ��������
��1.12 L(�ѻ���ɱ�״�����ٶ�����������ȫ���ݳ�)��
(1)��Һһ�����ڵ������� �����ܴ��ڵ������� ��
(2)ԭ��Һ��c(![]() )Ϊ ��c(
)Ϊ ��c(![]() ) c(
) c(![]() ) (���������=��)��
) (���������=��)��
(3)�������6�����Ӷ����ڣ���c(Cl) c(![]() ) (���������=��)��
) (���������=��)��
���𰸡�(1)![]() ��
��![]() ��
��![]() Cl��K+��Na+
Cl��K+��Na+
(2)0.05 mol/L ��
(3)��
����������ȡ��������Һ����BaCl2��Һ�а�ɫ�������ɣ��ټ�������������������ܽ⣬�����������ɣ�˵����ɫ����ΪBaCO3��BaSO4������һ����4.3 g������Һ�к���![]() ��
��![]() ��������м�����������ᣬ��2.33 g�������ܣ������ᱵ��������2.33 g��������������ӵ����ʵ�����
��������м�����������ᣬ��2.33 g�������ܣ������ᱵ��������2.33 g��������������ӵ����ʵ�����![]() =0.01 mol������̼�ᱵ��������4.3 g2.33 g=1.97 g��
=0.01 mol������̼�ᱵ��������4.3 g2.33 g=1.97 g��![]() �����ʵ�����
�����ʵ�����![]() =0.01 mol������������Һ�м���������NaOH��Һ�����ȣ�������ʹʪ���ɫʯ����ֽ�����������ǰ��������ʵ�����
=0.01 mol������������Һ�м���������NaOH��Һ�����ȣ�������ʹʪ���ɫʯ����ֽ�����������ǰ��������ʵ�����![]() =0.05 mol��˵����Һ����
=0.05 mol��˵����Һ����![]() �����ʵ�����0.05 mol���ۺ����Ϸ�����
�����ʵ�����0.05 mol���ۺ����Ϸ�����
(1)��Һһ�����ڵ������У�![]() ��
��![]() ��
��![]() �����ܴ��ڵ������У�Cl��K+��Na+��
�����ܴ��ڵ������У�Cl��K+��Na+��
(2)���ݼ���ó�c(![]() )=
)=![]() =0.05 mol/L��
=0.05 mol/L��![]() Ϊ0.01 mol��
Ϊ0.01 mol��![]() Ϊ0.05 mol����c(
Ϊ0.05 mol����c(![]() )��c(
)��c(![]() )��
)��
(3)������Һ������ԭ���趼���ڣ���ô0.05 mol+n(Na+)+n(K+)=2��0.01 mol+2��0.01 mol+n(Cl)���ݴ˵ó�n(Cl)=n(Na+)+n(K+)+0.01 mol��0.01 mol��


 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ʵ���ʵ��������������ý�����ȷ����
ʵ���ʵ����� | ���� | ʵ����� | |
A | �ô���ʯ�����ᷴӦ��ȡCO2���壬����ͨ��һ��Ũ�ȵ�Na2SiO3��Һ�� | ���ְ�ɫ���� | H2CO3�����Ա�H2SiO3������ǿ |
B | ��ij��Һ�ȵμ������ữ���ٵμ�BaCl2��Һ | �а�ɫ�������� | ԭ��Һ�к���SO42-��SO32-��HSO3-�е�һ�ֻ��� |
C |
| �Թ�b���Թ�a����Һ�ĺ�ɫ�� | ����Ӧ��Ũ�ȣ�ƽ��������Ӧ�����ƶ� |
D |
| ��������Ϊ��ɫ���ұ������Ϊ��ɫ | �����ԣ�Cl2>Br2>I2 |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��20��ʱ��H2S�ı�����Һ1L����Ũ��Ϊ0.1mol/L����Ҫʹ��Һ��pH�����ͬʱc(S2��)���ɲ�ȡ�Ĵ�ʩ��
A. ����������ˮ B. ����������NaOH����
C. ͨ��������SO2 D. ����������CuSO4����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������Ҫ�Ļ���ԭ�ϣ���������ˮâ��(Na2SO4)��̿�ۻ�ԭ���Ʊ���ԭ��Ϊ��Na2SO4��2CNa2S��2CO2��������Ҫ�������£�

(1) ���������в���ϡ��Һ������ˮ���ã�������________________________________________________________________________��
(2) ��֪��I2��2S2O32��===2I����S4O62�������Ƶõ�Na2S��9H2O�����к���Na2S2O3��5H2O�����ʡ�Ϊ�ⶨ��Ʒ�ijɷ֣���������ʵ�飬�������£�
a. ȡ����10.00 g���500.00 mL��Һ��
b. ȡ������Һ25.00 mL�ڵ���ƿ�У��������ZnCO3����Һ��ȥNa2S���ˣ�����Һ�е���2��3�ε�����Һ����0.050 00 mol��L��1 I2��Һ�ζ����յ㣬��ȥ5.00 mL I2��Һ��
c. ��ȡ������Һ25.00 mL�ڵ���ƿ�У�����50.00 mL 0.050 00 mol��L��1��I2��Һ��������2��3�ε�����Һ�����ñ�Na2S2O3��Һ�ζ������I2����ȥ15.00 mL 0.100 0 mol��L��1 Na2S2O3��Һ��
������b����ZnCO3��ȥNa2S�����ӷ���ʽΪ____________��
���жϲ���c�еζ��յ�ķ���Ϊ______________��
������������Na2S��9H2O��Na2S2O3��5H2O��������������������__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼ�л���˵����ȷ����

A. ���л���ķ���ʽΪC7H7O3
B. 1 mol���л���ֻ����2 mol H2�����ӳɷ�Ӧ
C. ���л�����һ���������ܷ���ȡ����������Ӧ
D. ���л����һ�ַ�����ͬ���칹���ܷ���������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ�У����ǻ��Ϸ�Ӧ����������ԭ��Ӧ����
A�������������������������2SO2+O2![]() 2SO3
2SO3
B������ͨ���廯����Һ�У�Cl2+2NaBr![]() 2NaCl+Br2
2NaCl+Br2
C��ϡ��������������Һ��Ϸ�Ӧ��HCl+AgNO3![]() AgCl��+HNO3
AgCl��+HNO3
D�������Ƹ�ˮ��Ӧ��Na2O+H2O![]() 2NaOH
2NaOH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������أ�K2FeO4����һ�����͡���Ч�����ˮ��������

��1����ҵ�ϵ�ʪ���Ʊ���������KClO��Fe��OH��3��KOH�������Ƶ�K2FeO4���÷�Ӧ�������뻹ԭ�����ʵ���֮��Ϊ________________��
��2��ʵ������ʳ�Ρ�����м�����ᡢKOH��Ϊԭ�ϣ�ͨ�����¹����Ʊ�K2FeO4��
�ٲ������ķ���Ϊ_________________������������ѹ���
�ڼ������X����ķ�����________________��
����������Һ�еõ�K2FeO4�������õ�ԭ����____________________��
��3���ⶨijK2FeO4��Ʒ������������ʵ�鲽�����£�
����1��ȷ����1.0g��Ʒ������100mL��Һ��
����2��ȷ��ȡ25.00mL K2FeO4��Һ���뵽��ƿ��
����3����ǿ������Һ�У��ù���CrO2����FeO42����Ӧ����Fe��OH��3��CrO42��
����4����ϡ���ᣬʹCrO42��ת��ΪCr2O72����CrO2��ת��ΪCr3+��Fe��OH��3ת��ΪFe3+
����5�������������������ָʾ������0.1000mol��L��1��NH4��2Fe��SO4��2����Һ�ζ����յ㣨��Һ���Ϻ�ɫ�����������ģ�NH4��2Fe��SO4��2��Һ���������3��ƽ��ʵ�飬ƽ�����ģ�NH4��2Fe��SO4��2��Һ�����30.00 mL��
��֪���ζ�ʱ�����ķ�ӦΪ��6Fe2+��Cr2O72����14H+=6Fe3+��2Cr3+��7H2O��
�ٲ���2��ȷ��ȡ25.00mL K2FeO4��Һ���뵽��ƿ�����õ�������______________��
��д������3�з�����Ӧ�����ӷ���ʽ__________________________��
�۲���5���ܷ�ָʾ��_________��ԭ����________________��
�ܸ�������ʵ�����ݣ��ⶨ����Ʒ��K2FeO4����������Ϊ__________��
��4������0.1mol��L-1��K2FeO4��������ҺpH������������ˮ��Һ�еĴ�����̬��ͼ��ʾ������˵����ȷ����__________ ������ĸ����
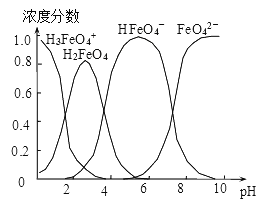
A��pH=2ʱ��c��H3FeO4+��+c��H2FeO4��+c��HFeO4����=0.1mol��L-1
B����pH��10��������Һ�м�����泥���HFeO4���ķֲ�����������
C����pH��1����Һ�м�HI��Һ��������Ӧ�����ӷ���ʽΪ��H2FeO4��H+��H3FeO4��
D����K2FeO4��������ˮ��ˮ��Һ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����14�֣���ҵ�Ͽ�����Ȼ��Ϊԭ���Ʊ��״���Ҳ����ˮú���ϳɼ״���
��1����֪2CH4��g����O2��g��=2CO2��g����4H2��g�� ��H��akJ/mol
CO��g����2H2��g����CH3OH��g����H��bkJ/mol
��д����CH4��O2��ȡ������Ȼ�ѧ����ʽ��___________________��
��2��������ͨ�����з�Ӧ�Ʊ��״���CO��g����2H2��g��![]() CH3OH��g����ͼ���Ƿ�ӦʱCO��g����CH3OH��g����Ũ����ʱ��t�ı仯������ӷ�Ӧ��ʼ��ƽ�⣬��CO��ʾƽ����Ӧ����v��CO����____________���÷�Ӧ��ƽ�ⳣ������ʽΪ______________��
CH3OH��g����ͼ���Ƿ�ӦʱCO��g����CH3OH��g����Ũ����ʱ��t�ı仯������ӷ�Ӧ��ʼ��ƽ�⣬��CO��ʾƽ����Ӧ����v��CO����____________���÷�Ӧ��ƽ�ⳣ������ʽΪ______________��

��3����һ�ݻ��ɱ���ܱ������г���10mol CO��20mol H2��CO��ƽ��ת�������¶ȣ�T����ѹǿ��P���ı仯����ͼ��ʾ��
������˵�������жϸ÷�Ӧ�ﵽ��ѧƽ��״̬����___________��������ĸ��
A��H2���������ʵ���CH3OH���������ʵ�2��
B��H2������������ٸı�
C����ϵ��H2��ת���ʺ�CO��ת�������
D����ϵ�������ƽ��Ħ���������ٸı�
���Ƚ�A��B����ѹǿ��СPA___________PB��������������=������
�����ﵽ��ѧƽ��״̬Aʱ�����������Ϊ20L�������Ӧ��ʼʱ�Գ���10molCO��20molH2������ƽ��״̬Bʱ���������V��B��=___________L��
��4���Լ״�Ϊȼ�ϣ�����Ϊ��������KOH��ҺΪ�������Һ�����Ƴ�ȼ�ϵ�أ��缫����Ϊ���Ե缫����
����KOH��Һ����������������Ӧ�����ӷ���ʽΪ_____________��
�����������Һ��KOH�����ʵ���Ϊ1��0mol������0��75mol�״����뷴Ӧʱ���������Һ�и������ӵ����ʵ���Ũ���ɴ�С��˳����______________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com