
������6�����ʣ�������������գ�
��Al ��FeCl3��Һ ��SO2 �ܼ�ʯ�� �ݰ��� ��NaOH����
(1)�ܵ������(����ţ�����ͬ) �����ڷǵ���ʵ��� ��
��������������� ��
(2)�������Һ��Ӧ�Ļ�ѧ����ʽΪ�� ��
(3)�����Һ�м���KI��Һ����CCl4��ȡ��CCl4�����ɫ��д���÷�Ӧ���̵����ӷ���ʽ�� ��
(4)ij��ѧʵ��С��ͬѧ��������װ���Ʊ��ݣ���̽���ݵ�����(������������ȥ)��

i��ʵ�����Ʊ��ݵĻ�ѧ����ʽΪ ��
ii���ռ���ʱ������ѡ������� (�a����b��)��
iii���Ź۲쵽װ��B�е���ƿ�ڲ����˺�ɫ��Ȫ����˵���ݾ��е������� ��
iv���������İ�������ͨ�����Һ�У��۲쵽�������� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ����
A.ͬһ�����Ԫ�أ�ԭ�Ӱ뾶Խ���䵥�ʵ��۵�һ��Խ��
B.ͬһ����Ԫ�ص�ԭ�ӣ��뾶ԽСԽ����ʧȥ����
C.ͬһ�����Ԫ�ص��⻯���Է�������Խ�����ķе�һ��Խ��
D.ϡ������Ԫ�ص�ԭ������Խ���䵥�ʵķе�һ��Խ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ�����Ʋ����е���(����)
A����Ũ��ˮ�м�����ʯ�ҿ�����ȡ������NH3
B����ˮ����һ���Լ����屽������������������
C������ͼ��ʾ��װ�òⶨ��ͭ(Cu��Zn�Ͻ�)��Zn�ĺ���
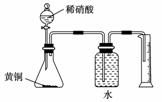
D����ȥ�����е�Ca2����Mg2����SO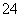 �����μ����������H2O��Ba(OH)2��Na2CO3��HCl
��������������H2O��Ba(OH)2��Na2CO3��HCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������Һ�пɴ������棬�γ���ɫ����Һ��һ����
A��K+��Na+��OH����Cl�� B��Ba2+��Cu2+��NO3����SO42��
C�� Mg2+��Na+��OH����SO42�� D��H+��Ba2+��NO3����OH��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ��ȷ����
A��������CO2ͨ�뱥��̼������Һ��: CO2��CO32����H2O==2HCO3��
B��FeSO4��Һ�ڿ����б��ʣ�4Fe2+��O2��2H2O==4Fe3+��4OH��
C�����������Һ��ͨ��������������Ca2+��ClO-��SO2��H2O==CaSO4����Cl-��2H��
D��̼����þ��Һ�м������ʯ��ˮ
Mg2++2HCO3��+2Ca2++4OH�� ==2CaCO3��+ Mg(OH)2��+ 2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������Ȼ�ѧ����ʽ�У��йئ�H�ıȽ���ȷ���� (����)
��CH4(g)��2O2(g)===CO2(g)��2H2O(g)����H1
CH4(g)��2O2(g)===CO2(g)��2H2O(l)����H2
��NaOH(aq)��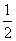 H2SO4(Ũ)===
H2SO4(Ũ)===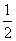 Na2SO4(aq)��H2O(l)����H3
Na2SO4(aq)��H2O(l)����H3
NaOH(aq)��CH3COOH(aq)===CH3COONa(aq)��H2O(l)����H4
A����H1����H2����H3����H4 B����H1����H2����H3����H4
C����H1����H2����H3����H4 D����H1����H2����H3����H4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ͼʾ��˵����ȷ����

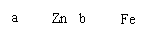
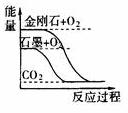

A��ͼ�ٱ�ʾʯīת��Ϊ���ʯ�����ȷ�Ӧ
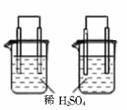
B��ͼ����ʾʵ��ɱȽϷǽ�����ǿ�����ȩ�̼����
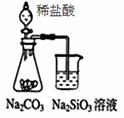
C��ͼ������NH3��������
D��ͼ����װ���н����缫a��b����������������жϽ�����ԣ�a��b
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ժȡѧ��ʵ�鱨�浥�е���䣬����˵����ȷ����(����)
A���ճ������в�������������������Ƿ���KIO3
B������м��Ũ��ˮ������Ͽ��Ƶ��屽
C����������ȥ�Ҵ��е�ˮ
D��ϴ�ӳ���ʱ��Ӧ�ò�����������裬ʹ�ó��������ϵ���������ˮ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
1.52g ͭþ�Ͻ���ȫ�ܽ���50mL �ܶ�Ϊ1.40 g/mL����������Ϊ63%��Ũ�����У��õ�NO2��N2O4�Ļ������1120 mL����״��������Ӧ�����Һ�м���1.0 mol/L NaOH��Һ������������ȫ������ʱ���õ�2.54 g����������˵������ȷ���ǣ�������
A���úϽ���ͭ��þ�����ʵ���֮����2:1
B. ��Ũ������HNO3�����ʵ���Ũ����14.0 mol/L
C��NO2��N2O4�Ļ�������У�NO2�����������80%
D���õ�2.54 g����ʱ������NaOH��Һ�������600 mLzxxk
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com