
����Ŀ����ˮ�Ǿ����Դ���⣬�Ӻ�ˮ����ȡʳ�κ���Ĺ������£�

��1��д������I�����ɵ�Ũ��Br2�����ӷ���ʽ______��
��2������I���ѻ��Br2������I���ֽ�Br2��ԭΪBr-����Ŀ��Ϊ������Ԫ�أ���д������II�Ļ�ѧ����ʽ_______��
��3����3mL��ˮ�м���1mL���Ȼ�̼�������ú۲쵽�Թ���ķֲ�����Ϊ��ͼ�е�_______��

��4��ij��ѧ�о���ѧϰС��Ϊ�˽�ӹ�ҵ�����ᴿ��ķ������������й����ϣ�Br2�ķе�Ϊ59�棬����ˮ���ж��Ժ�ǿ��ʴ�ԣ������������ͼװ�ü�ͼ���������������ۣ�
��C��Һ�������ɫΪ_____��
�������ӷ���ʽ����NaOHŨ��Һ������______��

���𰸡�Cl2+2Br-=2Cl-+Br2 Br2+SO2+2H2O=2HBr+H2SO4 D �����ɫ Br2+2OH-=Br-+BrO- +H2O
��������
��ˮ�����õ��Ȼ��ƣ�����Ȼ�����Һ������״̬�Ȼ��ƻ���������������ͨ��ĸҺ�з�����Ӧ�õ���Ũ�ȵ��嵥����Һ��ͨ���ȿ����������ö�������ˮ��Һ���յõ���HBr���������Һ��ͨ���������������õ��嵥�ʣ�������Ԫ�أ�����õ���ҵ�壻
(1)����I�����ɵ�Ũ��Br2���漰�����������ӵ��û���Ӧ��
(2)���������ֽ�Br2��ԭΪBr-�������������巢��������ԭ��Ӧ��
(3)���Ȼ�̼���ܶȱ�ˮ���������������Ȼ�̼��
(4)��ҵ�������ᴿ��ķ�������Ҫ��������������Ϊ����и�����ϢBr2�ķе���59�����ᴿ������ռ�59��ʱ����֣�C��Һ��Ϊ���������Ĵ��壬����ɫΪ���غ�ɫ���������ж�����Ҫ�ü�Һ�����ա�
��1�������ܹ����������ӵõ������Ӻ͵����壬���ӷ���ʽΪ��Cl2+2Br-=2Cl-+Br2��
�ʴ��ǣ�Cl2+2Br-=2Cl-+Br2��
��2������������л�ԭ�ԣ�����������ԣ����߷���������ԭ��Ӧ��������������ᣬ����II�Ļ�ѧ����ʽΪ��Br2+SO2+2H2O=2HBr+H2SO4��
�ʴ��ǣ�Br2+SO2+2H2O=2HBr+H2SO4��
��3�����Ȼ�̼�ܹ���ȡ��ˮ�е��壬�������Ȼ�̼���ܶȴ���ˮ���ܶȣ������������ǣ���Һ�ֲ㣬���ܽ����Ȼ�̼�гʳȺ�ɫ�������²�ʳȺ�ɫ���ϲ�Ϊˮ�㣬����ɫ����D��ȷ��
�ʴ�ѡD��
��4����C��Բ����ƿ�в���Һ��Ϊ�壬��ɫΪ�����ɫ��
����:�����ɫ��
��Br2�ж��������ŷŵ������У�D��ŨNaOH��Һ�����������ջӷ��������壬��Ӧ�����ӷ���ʽΪBr2+2OH-=Br-+BrO- +H2O��
�ʴ���: Br2+2OH-=Br-+BrO- +H2O��


 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2008��9�±�����Ӥ���̷�����Ⱦ�¼�������ʳ��������Ⱦ�̷۵�Ӥ����������ʯ��֢����ԭ���Ǹ��̷��к��������谷�������谷�ķ���ʽΪC3N3(NH2)3�������й������谷��˵������ȷ����
A.�����谷��C��N��H��ԭ�Ӹ�����Ϊ1��2��2
B.�����谷��C��N����Ԫ�ص�������Ϊ3��7
C.�����谷��Ħ������Ϊ126
D.�����谷�е�Ԫ�ص���������ԼΪ66.7��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����
A.���͡�ú�͡����;���������ʯ�͵ķ���
B.ú��Һ�����������������仯
C.��ϩ�ͱ�����ʹ���Ը��������Һ��ɫ
D.��Ȳ�ͱ�����ʹ��ˮ��ɫ��ԭ����ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���߷��ӻ�����H�ĺϳ�·�����£�

��֪��i. 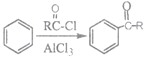
ii. 
�ش���������
(1)A�Ļ�ѧ����Ϊ___________��
(2)B��C�Ļ�ѧ����ʽΪ______________________��
(3)E�Ľṹ��ʽΪ___________����E����F�ķ�Ӧ����Ϊ______________________��
(4)G�й���������Ϊ___________����G��H�Ļ�ѧ����ʽΪ______________________��
(5)�����廯����L��G�Ĺ�������ͬ����L����Է���������GС28�����������������L��ͬ���칹����___________�֡�
����FeCl3��Һ������ɫ��Ӧ ����������3��ȡ����
(6)�� �л��ϳ�·�߿����Ʊ�
�л��ϳ�·�߿����Ʊ� ������������̺���֪��Ϣ������֪M��N�Ľṹ��ʽ�ֱ�Ϊ___________��___________��
������������̺���֪��Ϣ������֪M��N�Ľṹ��ʽ�ֱ�Ϊ___________��___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֮�乲��һ��̼ԭ�ӵĻ������Ϊ�ݻ��������[2��2]���飨![]() �������һ�֡����й��ڸû������˵���������
�������һ�֡����й��ڸû������˵���������
A. �뻷��ϩ��Ϊͬ���칹��
B. ���ȴ��ﳬ������
C. ����̼ԭ�Ӿ���ͬһƽ��
D. ����1 molC5H12������Ҫ2 molH2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ִ���ҵ�����Ȼ���Ϊԭ���Ʊ�������ֹ���������ͼ��

��1����ҵ�����У���ӦI�Ļ�ѧ����ʽ��______��
��2����ˮ�к��д�����NaCl���Լ�������Ca2+��Mg2+��SO42�����ӡ�
��Ϊ�õ������ı���NaCl��Һ�����������²������벹ȫ�������裺
a����Ũ����ĺ�ˮ�м������������������Һ���ˣ�
b������Һ��_______�����ˣ�
c������Һ��_______�����ˣ�
d������Һ�м���������ϡ���
e���������һ��ʱ�䣬�õ�����NaCl��Һ��
�ڲ���c�з�Ӧ�����ӷ���ʽ��____��
��3����ӦII�Ļ�ѧ����ʽ��______��
��4���ƵõĴ����к�������NaCl��ȡ5.5g������Ʒ��������ϡ���ᣬ�õ���״����1120mLCO2������Ʒ�д��������������______%������1λС������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��298K��1.01��105Pa��O2��S��Se��Te�ֱ���H2���ϵķ�Ӧ��������ͼ��ʾ��д��Se��H2���ϵ��Ȼ�ѧ��Ӧ����ʽ��ȷ����

A. Se(s)+ H2(g) = H2Se(g) ��H=-242kJ��mol-1
B. Se(s)+ H2(g) = H2Se(g) ��H=-20kJ��mol-1
C. Se(g)+ H2(g) = H2Se(g) ��H=+81kJ��mol-1
D. Se(s)+ H2(g) = H2Se(g) ��H=+81kJ��mol-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������ʵ���Ҫ���������ʽṹ����ش��������⣺
(1)��֪Ԫ��M���������Ca5(PO4)3F��һ��Ԫ�ء�Ԫ��M����̬ԭ�����ʧȥ��1������5��������������(�������ܣ��÷���I1��I5��ʾ)�����ʾ��
I1 | I2 | I3 | I4 | I5 | |
������ | 589.8 | 1145.4 | 4912.4 | 6491 | 8153 |
Ԫ��M����̬�������ϼ���_________�ۣ����̬ԭ�ӵ����Ų�ʽΪ_________��
(2)Ca3(PO4)3F�зǽ���Ԫ�ص縺���ɴ�С��˳��Ϊ_________��
(3)PO43-������ԭ�ӵ��ӻ���ʽΪ_________�������ӵĿռ乹��Ϊ_________������Ϊ________����ȵ�������_________ (��д������)��
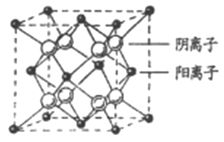
(4)CaF2�����ṹ��ͼ��ʾ����CaF2��������Ca2+����ҵȾ����Ca2+��ĿΪ_________����֪Ca2+��F�뾶�ֱ�Ϊa cm��b cm�������ӵ�����ΪNA��MΪĦ�������������ܶ�Ϊ________g��cm3(���ػ���)��
(5)��֪MgO��CaO�ľ���ṹ���ƣ���Ħ��Ӳ�ȵĴ�С��ϵΪ_________��ԭ��Ϊ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��aL�����Ϊ1��3��A��B���������Ļ�����壬����0.5aL(״̬��ͬ)���������ӳɷ�Ӧ����A��B����������ͨʽ������(����)
A. CnH2n��2��CnH2n��2B. C nH2n��CnH2n��2
C. CnH2n��CnH2nD. CnH2n��CnH2n��2
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com