
����һ��Ӧ�ù㷺�Ľ�������ҵ����Al2O3�ͱ���ʯ(Na3AlF6)������ڵ���Ƶá����������Ҫ�ɷ���Al2O3����������SiO2��Fe2O3��MgO����ҵ�ϴ�������������Al2O3����ȡ�����������£�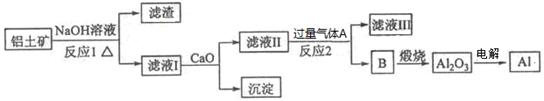
��ش��������⣺
��1������NaOH��Һ��Ӧ�����ӷ���ʽΪ ��
��2��ͼ���漰������Һ������IJ����������ձ�����������_________��
��3����Ӧ1�漰�Ļ�ѧ����ʽ�� �� ����Һ���м���CaO���ɵij�����_ (�ѧʽ)��
��4������Һ����ͨ������AΪ �����ɳ���B�����ӷ���ʽΪ ��
��1��2Al+2H2O+2OH��=2AlO2��+3H2�� ��2��©��
��3��Al2O3+2NaOH=2NaAlO2+H2O SiO2+2NaOH=Na2SiO3+H2O CaSiO3
��4��CO2 AlO2��+2H2O+CO2=Al(OH)3��+HCO3��
���������������1������NaOH��Һ��Ӧ�����ӷ���ʽΪ2Al+2H2O+2OH��=2AlO2��+3H2��;��2��ͼ���漰������Һ������IJ����������ձ�����������©������3�������������������Ʒ�Ӧ���ɹ�������ˮ����Ӧ����ʽΪ��2NaOH+SiO2�TNa2SiO3+H2O�����������������Ʒ�Ӧ����ƫ��������ˮ����Ӧ����ʽΪ��2NaOH+Al2O3�T2NaAlO2+H2O����Һ���к��й����ơ�ƫ�����ƣ�����CaO�������������ƣ���4���ɹ������̿�֪��BΪ����������������AΪ������̼����Һ����Ҫ��ƫ�����ƣ�ƫ��������Һͨ�������̼����������������̼�����ƣ������ӷ�Ӧ����ʽΪ��AlO2��+2H2O+CO2=Al(OH)3��+HCO3����
���㣺���ʷ�����ᴿ�ķ����ͻ��������ۺ�Ӧ��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������ͭ�Ƚ������仯�������ճ�������Ӧ�ù㷺,���������ʵ��ش�����:
(1)�����к���һ����̼������X(Fe3C)��X�������Ŀ����и�������,�����д��ԵĹ���Y,��Y���ڹ����������Һ�к��еĴ�������������������
(2)ij��Һ����Mg2+��Fe2+��Al3+��Cu2+����������,�����м��������NaOH��Һ��,����,�������������ղ������պ�Ĺ���Ͷ�������ϡ������,������Һ��ԭ��Һ���,��Һ�д������ٵ�����������������
A.Mg2+ B.Fe2+ C.Al3+ D.Cu2+
(3)����������Ҫ��ҵ����,�÷���м�Ʊ�������������:
�ش���������:
�ٲ������������������,���������������������
��д���ڿ���������FeCO3�Ļ�ѧ����ʽ������������������������������
(4)��Щͬѧ��ΪKMnO4��Һ�ζ�Ҳ�ܽ�����Ԫ�غ����IJⶨ��
a.��ȡ2.850 g�̷�(FeSO4��7H2O)��Ʒ,�ܽ�,��250 mL����ƿ�ж���;
b.��ȡ25.00 mL������Һ������ƿ��;
c.�������ữ��0.010 00 mol��L-1 KMnO4��Һ�ζ����յ�,����KMnO4��Һ�����ƽ��ֵΪ20.00 mL��
��ʵ��ǰ,����Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250 mL,����ʱ��Ҫ����������ƽ�����������ձ�����ͷ�ι���,������������
��ijͬѧ��Ƶ����еζ���ʽ,�����������������(�гֲ�����ȥ)(����ĸ���) 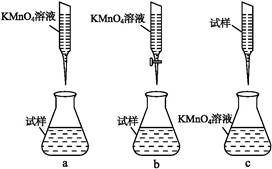
��д���ζ������з�Ӧ�����ӷ���ʽ:��������������������������������������
�ܼ���������Ʒ��FeSO4��7H2O����������Ϊ����������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ���Ѱ۲����ķ�Һ�к��д���FeSO4��H2SO4������Fe2(SO4)3��TiOSO4����������ⷨ������Ѫ�������������������������£�
��֪��TiOSO4������ˮ����ˮ�е���ΪTiO2+��SO42-����ش��������⣺
��1��д��TiOSO4ˮ����������H4TiO4�����ӷ���ʽ ��������м���������м��Ŀ���� ��
��2����ҵ����H4TiO4���Ƶ��Ѱ�TiO2��TiO2ֱ�ӵ�ԭ�������ŷ��������� ��һ�ֽ��Ƚ��ķ����������Ϊ���ڵ�CaCl2��ԭ����ͼ��ʾ�������ĵ缫��ӦΪ_______________��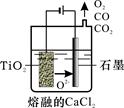
��3������ڵ����ӷ���ʽ�� �����ø���Ʒ��Ҫ ��__________���ѧʽ����
��4������ܵĽᾧ�����б������һ������նȣ�ԭ���� ��
��5�����������ϩ�����в���ϳɣ�
�����ϳ�·�ߵ��ܲ���Ϊ60%��������̼��������Ӧת��Ϊ������������IJ���Ϊ90%��������468 kg�����������壨M��234 g/mol����Ҫ��״���µ���ϩ m3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ú���Al2O3������Fe2O3��SiO2���������Ʊ���ˮ������Һ��ۺ����������������������£����ֲ����������ԣ���
I�����������м������H2SO4���ȡ����衢���ˡ�
II������Һ�м���һ������FeSO4��7H2O��˫��ˮ��
III������Һ�м���Ca(OH)2���壬������Һ��pHԼΪ1�����ˡ�
IV�������ȶ��������ȣ��õ���Ʒ��
��1��Fe2O3��H2SO4��Ӧ�����ӷ���ʽ��___________��
��2������I�й��˵õ��������ɷ���________���ѧʽ����
��3������I ��H2SO4��Ũ���뷴Ӧ�¶Ȼ�Ӱ���������Ľ����ʡ�������ͼ����������I ��H2SO4Ũ�ȵ����˷�Χ��__________����Ӧ�������¶���_________��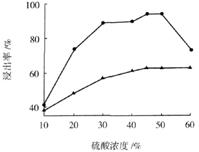

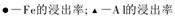
��4������II������n(Fe3+)�����ӷ���ʽ��_________��
��5������III�õ���ʽ��������[AlFe(OH)n(SO4)m]����Һ������II��Ӧ����n(Fe3+)��
n(Al3+)�sn(Fe3+)= ��
��6���о�������Һ��ۺ����������Ĵ���Խ�ߣ���ˮЧ��Խ�á���֪��
һЩ������20��ʱ���ܽ��
| ���� | Ca(OH)2 | CaSO4 | Na2SO4 |
| �ܽ��/g | 0.153 | 0.258 | 19.5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���к�FeCl2���ʵ��Ȼ�ͭ���壨CuCl2��2H2O����Ϊ��ȡ������CuCl2��2H2O�����Ƚ����Ƴ�ˮ��Һ��Ȼ����ͼ��ʾ��������ᴿ��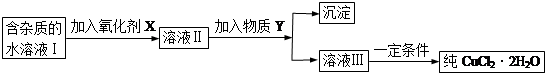

��֪H2O2��KMnO4��NaClO��K2Cr2O7������ǿ�����ԣ�Ҫ����Һ�е�Cu2����Fe2����Fe3������Ϊ�����������Һ��pH�ֱ�Ϊ6.4��6.4��3.7��
��ش��������� [��1��~��2��С������] ��
��1����ʵ�����ʺϵ�������X��__________
A��K2Cr2O7 B��NaClO C��H2O2 D��KMnO4
��2������Y��������___________
A��CuO B��CuCl2 C��Cu��OH��2 D��CuCO3
��3����YΪCu(OH)2��д����ȥFe3�������ӷ���ʽ��
��4��������������Ŀ����_______________________________________��
��5������ܲ���ֱ�������ᾧ�õ�CuCl2��2H2O��__________����ܡ����ܡ������粻�ܣ�Ӧ��β����������ܣ��˿ղ��_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ��ɫ����Һ�����ܺ�Al3����Fe3����Mg2����Na����CO32-��Cl����NO3-�������е������֡���������ʵ�飺
һ��ȡ��������Һ�������������ữ��AgNO 3��Һ���а�ɫ�������ɡ�
������ȡ������Һ�������������ƣ��а�ɫ���������������������Ƶ��������ɰ�ɫ��������������ͼ��ʾ��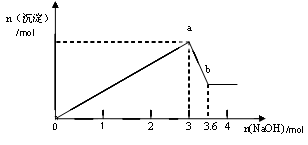
���ƶϣ�
��1������Һ��һ������__________ ____��һ��������________________��
��2����ͼ��֪��ɫ��������__________�֣��ֱ���__________________���ѧʽ���������ʵ�����Ϊ ��
��3�� д��ͼ��a b�仯���̵����ӷ���ʽ ��
b�仯���̵����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�й����ʼ�ת����ϵ����ͼ���Իش�
��1��ת���ٵķ�Ӧ����Ϊ .
��2��ת���ڵĻ�ѧ����ʽΪ .ת���۵����ӷ���ʽΪ .
��3����ת��������ʵ������Al(OH)3�����Լ�A��ѡ�� �������ƣ�.
��4����50 mL 3 mol��L-1 AlCl3��Һ�еμ�1 mol��L-1 NaOH��Һ�����Al3+������1/3ת��ΪAl(OH) 3������������NaOH��Һ���������Ϊ mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��.�ִ���ҵ�����Ȼ���Ϊԭ���Ʊ�������ֹ����������£�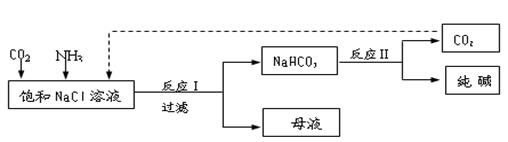 k+s-5#
k+s-5#
��֪NaHCO3�ڵ������ܽ�Ƚ�С����Ӧ��Ϊ��NaCl+CO2+NH3+H2O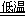 NaHCO3��+NH4Cl������ĸҺ�����ַ������£�
NaHCO3��+NH4Cl������ĸҺ�����ַ������£�
��1����ĸҺ�м���ʯ���飬�ɽ�����________ѭ�����á�
��2����ĸҺ��ͨ��NH3������ϸС��ʳ�ο��������£��ɵõ�NH4Cl���塣��д��ͨ��NH3���ܽ�Ƚ�С����ʽ̼����ת��Ϊ�ܽ�Ƚϴ��̼���ε����ӷ���ʽ _____________________________��
��.ij��ѧС��ģ�⡰�����Ƽ������NaCl��NH3��CO2��ˮ��Ϊԭ���Լ���ͼ��ʾװ����ȡNaHCO3��Ȼ���ٽ�NaHCO3�Ƴ�Na2CO3��
��1��װ�ñ�����ˮ�������� ��
��2����װ�ñ��в�����NaHCO3��ȡNa2CO3ʱ����Ҫ���е�ʵ�������_______��ϴ�ӡ����ա�NaHCO3ת��ΪNa2CO3�Ļ�ѧ����ʽΪ ��
��3�����ڣ�2�������յ�ʱ��϶̣�NaHCO3���ֽⲻ��ȫ����С���һ�ݼ�����t1 min��NaHCO3 ��Ʒ����ɽ���������̽����ȡ������t1 min��NaHCO3��Ʒ29.6 g��ȫ����ˮ�Ƴ���Һ��Ȼ�������Һ�л����صμ�ϡ���ᣬ�����Ͻ��衣��������ļ��룬��Һ���й����ӵ����ʵ����ı仯��ͼ��ʾ��������a��Ӧ����Һ�е�������___________�������ӷ�����ͬ��������c��Ӧ����Һ�е�������___________������Ʒ��NaHCO3��Na2CO3�����ʵ���֮���� ��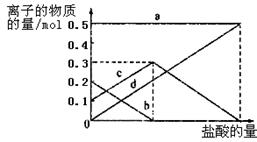
��4����ȡ21.0 g NaHCO3���壬������t2 rnin��ʣ����������Ϊl4.8 g������Ѵ�ʣ�����ȫ�����뵽200 mL 2 mol?L��1�������У����ַ�Ӧ����Һ��H+ �����ʵ���Ũ��Ϊ____________������Һ����仯���Բ��ƣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������A�����ᷴӦ����dz��ɫ��ҺB��ͬʱ�ų�����C�������B��Һ��ͨ����������Bת����ػ�ɫ��ҺD������ҺD��Ϊ���ݣ�һ�ݼ��뼸��KSCN��Һ����Һ���Ѫ��ɫ����һ�ݼ���A�����ػ�ɫ��ҺD���±��dz��ɫ��ҺB����
��1�������ʵĻ�ѧʽ�ֱ��ǣ�A________ ��B________ ��C________ ��D________��
��2���йط�Ӧ�����ӷ���ʽ�У�
�� B��D___________________________��
�� D��B____________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com