
УРТ»ОЮЙ«НёГчИЬТєЈ¬їЙДЬє¬Al3Ј«ЎўFe3Ј«ЎўMg2Ј«ЎўNaЈ«ЎўCO32-ЎўClЈЎўNO3-µИАлЧУЦРµДИфёЙЦЦЎЈПЦЧцИзПВКµСйЈє
Т»ЎўИЎЙЩБїёГИЬТєЈ¬µОИлУГПхЛбЛб»ЇµДAgNO 3ИЬТєЈ¬УР°ЧЙ«іБµнЙъіЙЎЈ
¶юЎўБнИЎІї·ЦИЬТєЈ¬јУИлЗвСх»ЇДЖЈ¬УР°ЧЙ«іБµнІъЙъЈ¬јУИлЗвСх»ЇДЖµДБїУлЙъіЙ°ЧЙ«іБµнµДБїїЙУГПВНј±нКѕЎЈ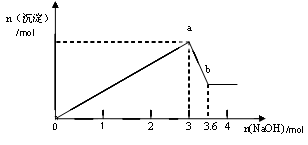
КФНЖ¶ПЈє
ЈЁ1Ј©ёГИЬТєЦРТ»¶ЁґжФЪ__________ ____Ј¬Т»¶ЁІ»ґжФЪ________________ЎЈ
ЈЁ2Ј©УЙНјїЙЦЄ°ЧЙ«іБµн№ІУР__________ЦЦЈ¬·Ц±рКЗ__________________ЈЁМо»ЇС§КЅЈ©Ј¬ЖдОпЦКµДБї±ИОЄ ЎЈ
ЈЁ3Ј© РґіцНјЦРa b±д»Ї№эіМµДАлЧУ·ЅіМКЅ ЎЈ
b±д»Ї№эіМµДАлЧУ·ЅіМКЅ ЎЈ
ЈЁ1Ј©Al3Ј«ЎўMg2Ј«ЎўClЈ Ј» Fe3Ј«ЎўCO32-
ЈЁ2Ј©2Ј» Al(OH)3ЎўMg(OH)2 Ј» 1:1
ЈЁ3Ј©Al(OH)3+ OHЈ AlO2Ј+ 2H2O
AlO2Ј+ 2H2O
ЅвОцКФМв·ЦОцЈєУЙУЪИЬТєКЗОЮЙ«НёГчµДЈ¬ЛщТФЈ¬КЧПИЕЕіэFe3Ј«µДґжФЪЈ¬ТтОЄFe3Ј«µДИЬТєіКЧШ»ЖЙ«ЎЈИЎЙЩБїёГИЬТєЈ¬µОИлУГПхЛбЛб»ЇµДAgNO 3ИЬТєЈ¬УР°ЧЙ«іБµнЙъіЙЈ¬ЛµГчёГИЬТєЦРТ»¶ЁґжФЪClЈЈ¬ТтОЄЦ»УРAgCl°ЧЙ«ИЬТєКЗІ»ИЬУЪПЎHNO3µДЎЈБнИЎІї·ЦИЬТєЈ¬јУИлЗвСх»ЇДЖИЬТєЈ¬ПЦПуОЄПИУРіБµнЈ¬єуіБµнПыК§Ј¬ЛµГчФИЬТєЦРТ»¶ЁґжФЪAl3Ј«ЎўMg2Ј«Ј¬Н¬К±ЕЕіэБЛCO32-µДґжФЪЈ¬ТтОЄAl3Ј«ЎўMg2Ј«УлCO32--»б·ґУ¦Ј¬І»ДЬґуБї№ІґжЎЈ¶шNaЈ«УлNO3-ґжІ»ґжФЪ¶ј¶ФХыёцПЦПуОЮУ°ПмЎЈµ±іБµнµДБїґпµЅЧоёЯК±Ј¬ФЩјУИлЗвСх»ЇДЖИЬТєЈ¬іБµнІї·ЦПыК§Ј¬ЖдФТтКЗЙъіЙµДЗвСх»ЇВБіБµнКЗБЅРФЗвСх»ЇОпЈ¬ДЬУлЗвСх»ЇДЖИЬТєјМРш·ґУ¦ЙъіЙЖ«ВБЛбДЖєНЛ®Ј¬¶шЗвСх»ЇГѕіБµнФтІ»ДЬУлЗвСх»ЇДЖИЬТє·ґУ¦ЎЈ
їјµгЈєОпЦКНЖ¶ПЎЈ



| Дкј¶ | ёЯЦРїОіМ | Дкј¶ | іхЦРїОіМ |
| ёЯТ» | ёЯТ»Гв·СїОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СїОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СїОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СїОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СїОіМНЖјцЈЎ | іхИэ | іхИэГв·СїОіМНЖјцЈЎ |
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈєМоїХМв
CuSO4Ў¤5H2OКЗНµДЦШТЄ»ЇєПОпЈ¬УРЧЕ№г·єµДУ¦УГЎЈТФПВКЗCuSO4Ў¤5H2OµДКµСйКТЦЖ±ёБчіМНјЎЈ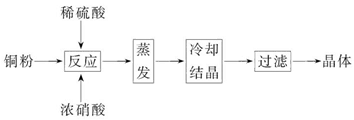
ёщѕЭМвТвНкіЙПВБРМоїХЈє
(1)Птє¬Н·ЫµДПЎБтЛбЦРµОјУЕЁПхЛбЈ¬ФЪН·ЫИЬЅвК±їЙТФ№ЫІмµЅµДКµСйПЦПуЈє_____________Ўў_____________ЎЈ
(2)Из№ыН·ЫЎўБтЛбј°ПхЛб¶ј±ИЅПґїѕ»Ј¬ФтЦЖµГµДCuSO4Ў¤5H2OЦРїЙДЬґжФЪµДФУЦККЗ_____________Ј¬іэИҐХвЦЦФУЦКµДКµСйІЩЧчіЖОЄ_____________ЎЈ
(3)ТСЦЄЈєCuSO4+2NaOH=Cu(OH)2Ўэ+Na2SO4ЎЈіЖИЎ0.100 0 gМбґїєуµДCuSO4Ў¤5H2OКФСщУЪЧ¶РОЖїЦРЈ¬јУИл0.100 0 mol/LЗвСх»ЇДЖИЬТє28.00 mLЈ¬·ґУ¦НкИ«єуЈ¬№эБїµДЗвСх»ЇДЖУГ
0.100 0 mol/LСОЛбµО¶ЁЦБЦХµгЈ¬єДУГСОЛб20.16 mLЈ¬Фт0.100 0 gёГКФСщЦРє¬CuSO4Ў¤5H2O_____________gЎЈ
(4)ЙПКцµО¶ЁЦРЈ¬µО¶Ё№ЬФЪЧўИлСОЛбЦ®З°Ј¬ПИУГХфБуЛ®Пґѕ»Ј¬ФЩУГ_____________ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈєМоїХМв
ЈЁ8·ЦЈ©РїєНВБ¶јКЗ»оЖГЅрКфЈ¬ЖдЗвСх»ЇОпјИДЬИЬУЪЗїЛбЈ¬УЦДЬИЬУЪЗїјоЎЈµ«КЗЗвСх»ЇВБІ»ИЬУЪ°±Л®Ј¬ЗвСх»ЇРїИЬУЪ°±Л®ЙъіЙZnЈЁNH3Ј©22Ј«ЎЈ»ШґрПВБРОКМвЈє
ЈЁ1Ј©µҐЦКВБИЬУЪЗвСх»ЇДЖИЬТєєуЈ¬ИЬТєЦРВБФЄЛШµДґжФЪРОКЅОЄ ЈЁУГ»ЇС§КЅ±нґпЈ©ЎЈ ЈЁ2Ј©РґіцРїєНЗвСх»ЇДЖИЬТє·ґУ¦µД»ЇС§·ЅіМКЅ ЎЈ
ЈЁ3Ј©ПВБРёчЧйЦРµДБЅЦЦИЬТєЈ¬УГП໥µОјУµДКµСй·Ѕ·ЁјґїЙјш±рµДКЗ ЎЈ
ўЩБтЛбВБєНЗвСх»ЇДЖ ўЪБтЛбВБєН°±Л® ўЫБтЛбРїєНЗвСх»ЇДЖ ўЬБтЛбРїєН°±Л®
ЈЁ4Ј©РґіцїЙИЬРФВБСОУл°±Л®·ґУ¦µДАлЧУ·ЅіМКЅ____________________________________________ЎЈ КФЅвКНФЪКµСйКТІ»ККТЛУГїЙИЬРФРїСОУл°±Л®·ґУ¦ЦЖ±ёЗвСх»ЇРїµДФТт___________________________ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈєМоїХМв
№¤ТµЙПіЈУГМъЦКИЭЖчКўЧ°АдЕЁБтЛбЎЈОЄСРѕїМъЦКІДБПУлИИЕЁБтЛбµД·ґУ¦Ј¬ДіС§П°РЎЧйЅшРРБЛТФПВМЅѕї»о¶ЇЈє
ЈЁ1Ј©Ѕ«ТСИҐіэ±нГжСх»ЇОпµДМъ¶¤ЈЁМјЛШёЦЈ©·ЕИлАдЕЁБтЛбЦРЈ¬10·ЦЦУєуТЖИлБтЛбНИЬТєЦРЈ¬Ж¬їМєуИЎіц№ЫІмЈ¬Мъ¶¤±нГжОЮГчПФ±д»ЇЈ¬ЖдФТтКЗ__________ ______________________________________ЎЈ
ЈЁ2Ј©БніЖИЎМъ¶¤6Ј®0g·ЕИл15Ј®0mlЕЁБтЛбЦРЈ¬јУИИЈ¬ід·ЦУ¦єуµГµЅИЬТєXІўКХјЇµЅЖшМеYЎЈ
ўЩјЧН¬С§ИПОЄXЦРіэFe3+Нв»№їЙДЬє¬УРFe2+ЎЈРґіцЙъіЙFe2+ЛщУРїЙДЬµДАлЧУ·ґУ¦·ЅіМКЅЈє ЎЈ
ИфТЄИ·ИПЖдЦРУРFe2+Ј¬У¦СЎУГ ЈЁСЎМоРтєЕЈ©ЎЈ
aЈ®KSCNИЬТєєНВИЛ® bЈ®Мъ·ЫєНKSCNИЬТє
cЈ®ЕЁ°±Л® dЈ®ЛбРФKMnO4ИЬТє
ўЪТТН¬С§ИЎ336mlЈЁ±кЧјЧґїцЈ©ЖшМеYНЁИлЧгБїдеЛ®ЦРЈ¬·ўЙъ·ґУ¦µД»ЇС§·ЅіМКЅОЄЈє ЎЈ
И»єујУИлЧгБїBaCl2ИЬТєЈ¬ѕККµ±ІЩЧчєуµГёЙФп№ММе2Ј®33gЎЈУЙУЪґЛНЖЦЄЖшМеYЦРSO2µДМе»э·ЦКэОЄ ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈєМоїХМв
ВБКЗТ»ЦЦУ¦УГ№г·єµДЅрКфЈ¬№¤ТµЙПУГAl2O3єН±щѕ§КЇ(Na3AlF6)»мєПИЫИЪµзЅвЦЖµГЎЈВБНБїуµДЦчТЄіЙ·ЦКЗAl2O3Ј¬є¬УРФУЦКSiO2ЎўFe2O3ЎўMgOЎЈ№¤ТµЙПґУВБНБїуЦРМбБ¶Al2O3ІўЦЖИЎВБµДБчіМИзПВЈє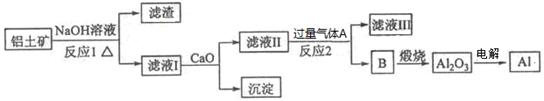
Зл»ШґрПВБРОКМвЈє
ЈЁ1Ј©ВБУлNaOHИЬТє·ґУ¦µДАлЧУ·ЅіМКЅОЄ ЎЈ
ЈЁ2Ј©НјЦРЙжј°·ЦАлИЬТєУліБµнµДІЈБ§ТЗЖчУРЙХ±ЎўІЈБ§°фЎў_________ЎЈ
ЈЁ3Ј©·ґУ¦1Йжј°µД»ЇС§·ЅіМКЅУР Ўў ЎЈВЛТєўсЦРјУИлCaOЙъіЙµДіБµнКЗ_ (Мо»ЇС§КЅ)ЎЈ
ЈЁ4Ј©ПтВЛТєўтЦРНЁИлЖшМеAОЄ Ј¬ЙъіЙіБµнBµДАлЧУ·ЅіМКЅОЄ ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈєМоїХМв
FeCl3ФЪПЦґъ№¤ТµЙъІъЦРУ¦УГ№г·єЎЈДі»ЇС§СРѕїРФС§П°РЎЧйДЈД⹤ҵЙъІъБчіМЦЖ±ёОЮЛ®FeCl3Ј¬ФЩУГё±ІъЖ·FeCl3ИЬТєОьКХУР¶ѕµДH2SЎЈ
ўс.ѕІйФДЧКБПµГЦЄЈєОЮЛ®FeCl3ФЪїХЖшЦРТЧі±ЅвЈ¬јУИИТЧЙэ»ЄЎЈЛыГЗЙијЖБЛЦЖ±ёОЮЛ®FeCl3µДКµСй·Ѕ°ёЈ¬Ч°ЦГКѕТвНјЈЁјУИИј°јРіЦЧ°ЦГВФИҐЈ©ј°ІЩЧчІЅЦиИзПВЈє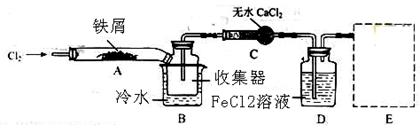
ўЩјмІйЧ°ЦГµДЖшГЬРФЈ»
ўЪНЁИлёЙФпµДCl2Ј¬ёПѕЎЧ°ЦГЦРµДїХЖшЈ»
ўЫУГѕЖѕ«µЖФЪМъРјПВ·ЅјУИИЦБ·ґУ¦НкіЙ
ўЬЈ®Ј®Ј®Ј®Ј®Ј®Ј®Ј®
ўЭМеПµАдИґєуЈ¬НЈЦ№НЁИлCl2Ј¬ІўУГёЙФпµДN2ёПѕЎCl2Ј¬Ѕ«КХјЇЖчГЬ·в
Зл»ШґрПВБРОКМвЈє
ЈЁ1Ј©Ч°ЦГAЦР·ґУ¦µД»ЇС§·ЅіМКЅОЄ ЎЈ
ЈЁ2Ј©µЪўЫІЅјУИИєуЈ¬ЙъіЙµДСМЧґFeCl3ґуІї·ЦЅшИлКХјЇЖчЈ¬ЙЩБїіБ»эФЪ·ґУ¦№ЬAУТ¶ЛТЄК№іБ»эµДFeCl3ЅшИлКХјЇЖчЈ¬µЪўЬІЅІЩЧчКЗ ЎЈ
ЈЁ3Ј©ІЩЧчІЅЦиЦРЈ¬ОЄ·АЦ№FeCl3і±ЅвЛщІЙИЎµДґлК©УРЈЁМоІЅЦиРтєЕЈ© ЎЈ
ЈЁ4Ј©Ч°ЦГDЦРFeCl2И«Ії·ґУ¦єуЈ¬ТтК§ИҐОьКХCl2µДЧчУГ¶шК§Р§Ј¬РґіцјмСйFeCl2КЗ·сК§Р§µДКФјБЈє ЎЈ
ЈЁ5Ј©ФЪРйПЯїтЦР»іцОІЖшОьКХЧ°ЦГEІўЧўГчКФјБЎЈ
ўтЈ®ёГЧйН¬С§УГЧ°ЦГDЦРµДё±ІъЖ·FeCl3ИЬТєОьКХH2SЈ¬µГµЅµҐЦКБтЈ»№эВЛєуЈ¬ФЩТФКЇД«ОЄµзј«Ј¬ФЪТ»¶ЁМхјюПВµзЅвВЛТєЎЈ
ЈЁ6Ј©FeCl3УлH2S·ґУ¦µДАлЧУ·ЅіМКЅОЄ ЎЈ
ЈЁ7Ј©µзЅвіШЦРH+ФЪТхј«·ЕµзІъЙъH2Ј¬Сфј«µДµзј«·ґУ¦КЅОЄ ЎЈ
ЈЁ8Ј©ЧЫєП·ЦОцКµСйўтµДБЅёц·ґУ¦Ј¬їЙЦЄёГКµСйУРБЅёцПФЦшУЕµгЈє
ўЩH2SµДФЧУАыУГВКОЄ100%Ј»ўЪ ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈєМоїХМв
іЈјыЅрКфµҐЦКAЎўBєН·ЗЅрКфµҐЦКCЎўDТФј°ЛьГЗ»ЇєПОпЦ®јдµДЧЄ»Ї№ШПµИзПВЎЈFЎўJјИДЬИЬУЪЗїЛбMУЦДЬИЬУЪЗїјоNЈ¬ZµДД¦¶ыЦКБїОЄ198 gЎ¤molЈ1Ј¬ЗТЖдЦРёчФЄЛШµДЦКБї±ИОЄЈєјШ:ЅрКфB:СхЈЅ39:28:32ЎЈ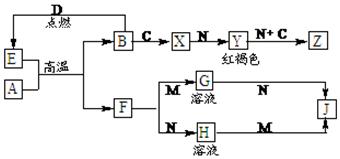
Зл»ШґрПВБРОКМвЈє
ЈЁ1Ј©CµД»ЇС§КЅОЄ Ј¬ZµД»ЇС§КЅОЄ ЎЈ
ЈЁ2Ј©РґіцјмСйXЦРСфАлЧУµД·Ѕ·Ё ЎЈ
ЈЁ3Ј©РґіцEєНAФЪёЯОВПВ·ґУ¦µД»ЇС§·ЅіМКЅОЄ ЎЈ
ЈЁ4Ј©РґіцAєНN·ґУ¦µДАлЧУ·ЅіМКЅ ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈєМоїХМв
УГє¬УРA12O3ЎўSiO2єНЙЩБїFeO·xFe2O3µДВБ»ТЦЖ±ёA12(SO4)3·18H2OЈ¬№¤ТХБчіМИзПВ(Ії·ЦІЩЧчєНМхјюВФ)Јє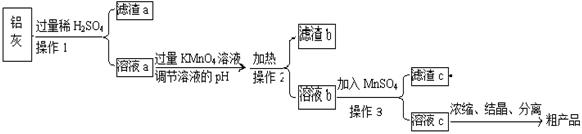
ЈЁ1Ј©ВЛФьaїЙУГУЪЦЖФм ЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎ
ЈЁ2Ј©ЗлЅ«MnO4ЈСх»ЇFe2+µДАлЧУ·ЅіМКЅІ№ідНкХыЈє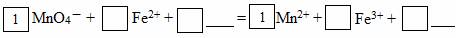
Иф·ґУ¦ЦРЧЄТЖБЛ2molµзЧУЈ¬ФтПыєДСх»ЇјБµДОпЦКµДБїОЄ molЎЈЎЎЎЎ
ЈЁ3Ј©ТСЦЄЙъіЙЗвСх»ЇОпіБµнµДpHИзПВЈє
| | Al(OH)3 | Fe(OH)2 | Fe(OH)3 |
| їЄКјіБµнК± | 3.4 | 6.3 | 1.5 |
| НкИ«іБµнК± | 4.7 | 8.3 | 2.8 |
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈєМоїХМв
ґУВБНБїуЈЁЦчТЄіЙ·ЦКЗ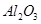 Ј¬є¬
Ј¬є¬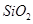 Ўў
Ўў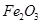 ЎўMgOµИФУЦКЈ©ЦРМбИЎБЅЦЦ№¤ТХЖ·µДБчіМИзПВЈє
ЎўMgOµИФУЦКЈ©ЦРМбИЎБЅЦЦ№¤ТХЖ·µДБчіМИзПВЈє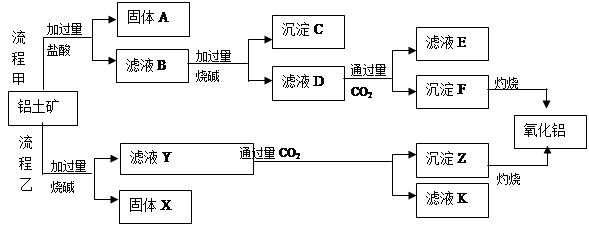
Зл»ШґрПВБРОКМвЈє
ЈЁ1Ј©БчіМТТјУИлЙХјоєуµДАлЧУ·ЅіМКЅОЄ_________________________________________.
ЈЁ2Ј©№ММеAµДУ¦УГ_________________________________________.ЈЁБЅµгЈ©
ЈЁ3Ј©ВЛТєDУлЙЩБїCO2·ґУ¦µДАлЧУ·ЅіМКЅОЄ__________________________________Ј¬
ПтёГВЛТєKЦРјУИлЧгБїКЇ»ТЛ®µДАлЧУ·ЅіМКЅКЗ________
ЈЁ4Ј©БчіМТТЦЖСх»ЇВБµДУЕµгКЗЛщУГµДКФјБЅПѕјГЈ¬И±µгКЗ__________________________
ЈЁ5Ј©ТСЦЄ298KК±Ј¬ µДИЬ¶И»эіЈКэ
µДИЬ¶И»эіЈКэ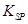 =10-11,ИЎККБїµДВЛТєB,јУИлТ»¶ЁБїµДЙХјоЗЎК№ГѕАлЧУіБµнНкИ«Ј¬ФтИЬТєµДPHЧоРЎОЄ_______.
=10-11,ИЎККБїµДВЛТєB,јУИлТ»¶ЁБїµДЙХјоЗЎК№ГѕАлЧУіБµнНкИ«Ј¬ФтИЬТєµДPHЧоРЎОЄ_______.
Ійїґґр°ёєНЅвОц>>
№ъјКѧУУЕСЎ - Б·П°ІбБР±н - КФМвБР±н
єю±±КЎ»ҐБЄНшОҐ·ЁєНІ»БјРЕПўѕЩ±ЁЖЅМЁ | НшЙПУРє¦РЕПўѕЩ±ЁЧЁЗш | µзРЕХ©ЖѕЩ±ЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРє¦РЕПўѕЩ±ЁЧЁЗш | ЙжЖуЗЦИЁѕЩ±ЁЧЁЗш
ОҐ·ЁєНІ»БјРЕПўѕЩ±Ёµз»°Јє027-86699610 ѕЩ±ЁУКПдЈє58377363@163.com