
����Ŀ����VA��Ԫ��������������������Ҫ��;���ش���������:
��1������������(�׳ơ����ơ�������ʳƷ������,��ˮ�ֱ��ּ���Ʒ�ʸ������ȡ�
������Ľṹʽ��ͼ��ʾ,����Ҫ�ĵ��뷽��ʽΪ______________��
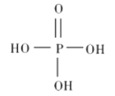
������������������������ȥ������ˮ��IJ���,���������ƵĻ�ѧʽΪ_______________��
��2���ڼ��������£���������(H2PO2-)�����ڻ�ѧ������д���䷴Ӧ�����ӷ���ʽ______________��(���������뻹ԭ�������ʵ���֮��Ϊ1:4)
��3���ɹ�ҵ����(�������顢����þ��)�Ʊ��ߴ�����(�۵�44��,�е�280��),��Ҫ������������:
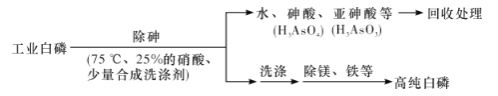
�ٳ��������75 ���½���,�������ԭ����____________(����ĸ����)��
a,ʹ�����ۻ�,������ˮ b.���Ͱ��Ķ���
c.�¶Ȳ��˹���,��ֹ����ֽ� d.�ʵ�����¶�,����Ӧ����
��������������ʱ����ԭΪNO,д����ת��Ϊ������Ļ�ѧ����ʽ:______________________________��
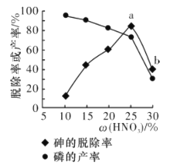
��ij������,��һ���������ᴦ��һ�����Ĺ�ҵ����,����ѳ��ʼ��IJ������������������ı仯��ͼ��ʾ,����ѳ��ʴ�a�㵽b�㽵�͵�ԭ����__________________��
��4��������������Һ�д���ƽ��:Ag+(aq) + 2NH3(aq) ![]() Ag(NH3)2+(aq),K=l.10��107 ;��֪������Ksp(AgCl)=1.45��10-10������淴ӦAgCl(s) +2NH3(aq)
Ag(NH3)2+(aq),K=l.10��107 ;��֪������Ksp(AgCl)=1.45��10-10������淴ӦAgCl(s) +2NH3(aq)![]() Ag(NH3)2+(aq)+Cl-(aq)�Ļ�ѧƽ�ⳣ��K=_________(����2λ��Ч����)��1L1mol/L��ˮ���������ܽ�AgCl ______mol(����1λ��Ч����)��
Ag(NH3)2+(aq)+Cl-(aq)�Ļ�ѧƽ�ⳣ��K=_________(����2λ��Ч����)��1L1mol/L��ˮ���������ܽ�AgCl ______mol(����1λ��Ч����)��
���𰸡�![]() Na5P3O10
Na5P3O10 ![]() cd
cd ![]() ����Ũ�ȴ�������ǿ���н϶�����������������ף������ʵ� 1.6��10-3 0.04
����Ũ�ȴ�������ǿ���н϶�����������������ף������ʵ� 1.6��10-3 0.04
��������
��1������������Ԫ���ᣬ�ֲ����룬��Ҫ�ĵ��뷽��ʽΪ![]() ��
��
������������������������ȥ������ˮ��IJ������ԭ���غ��֪��������ķ���ʽ��H5P3O10�������������ǻ�����ԭ�ӱ�������ȡ���������������ƣ����������ƵĻ�ѧʽΪ��Na5P3O10��
��2����ѧ����![]() �����������뻹ԭ�������ʵ���֮��Ϊ1:4������PԪ�ػ��ϼ���+1����Ϊ+5��H2PO2�C������Ϊ
�����������뻹ԭ�������ʵ���֮��Ϊ1:4������PԪ�ػ��ϼ���+1����Ϊ+5��H2PO2�C������Ϊ![]() �����ݵ�ʧ�����غ㣬��Ӧ�����ӷ���ʽ��
�����ݵ�ʧ�����غ㣬��Ӧ�����ӷ���ʽ��![]() ��
��
��3����a.���ײ�����ˮ����a����b.�ۻ����ܸı��䶾�ԣ���b����c.�¶ȹ��ߣ������ֽ⣬��Ҫ���ƺ��ʵ��¶ȣ���c��ȷ��d.����¶ȿ��Լӿ췴Ӧ���ʣ���d��ȷ��ѡcd��
�������������Ϊ�����ᣬ��Ԫ�ػ��ϼ���0����Ϊ+3��NԪ�ػ��ϼ���+5����Ϊ+2�����ݵ�ʧ�����غ㣬��ѧ����Ϊ![]() ��
��
������Ũ�ȴ�������ǿ����a�㵽b�㣬�н϶�����������������ף����������ʵͣ�
��4��Ag+(aq) + 2NH3(aq) ![]() Ag(NH3)2+(aq)��K=l.10��107��
Ag(NH3)2+(aq)��K=l.10��107�� 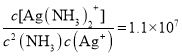 ��Ksp(AgCl)=1.45��10-10����
��Ksp(AgCl)=1.45��10-10����![]() ���淴ӦAgCl(s) +2NH3(aq)
���淴ӦAgCl(s) +2NH3(aq)![]() Ag(NH3)2+(aq)+Cl-(aq)�Ļ�ѧƽ�ⳣ��
Ag(NH3)2+(aq)+Cl-(aq)�Ļ�ѧƽ�ⳣ��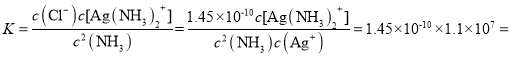 1.6��10-3��
1.6��10-3��
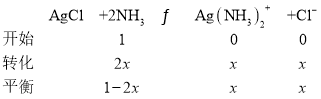
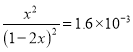 ��x=0.04��1L1mol/L��ˮ���������ܽ�AgCl 0.04mol��
��x=0.04��1L1mol/L��ˮ���������ܽ�AgCl 0.04mol��


 ��ְٷְټ���ϵ�д�
��ְٷְټ���ϵ�д� �����ƻ���ĩ��̶�100��ϵ�д�
�����ƻ���ĩ��̶�100��ϵ�д� �ܿ���ȫ��100��ϵ�д�
�ܿ���ȫ��100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������������ɵļ���ȼ�ϵ��ԭ����ͼ��ʾ������˵����ȷ����
A.�������Һ�е�����������
B.��ظ�����ӦΪ��2NH3-6e-=N2+6H+
C.������ͨ�����������ͬ���������֮��Ϊ15: 4 (���������O2�������Ϊ20%)
D.�õ�ظ�Ǧ���س�磬ȼ�ϵ��������Ӧl molO2��Ǧ������2mol PbSO4������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��100�����¶���(�����漰����Һ�¶Ⱦ�Ϊ100��)��ˮ�����ӻ�KW��1��10��12������˵����ȷ����
A.0.001 mol/L��NaOH��ҺpH��9
B.0.1 mol/L��H2SO4��ҺpH��1
C.0.005 mol/L��H2SO4��Һ��0.01 mol/L��NaOH��Һ�������ϣ������ҺpHΪ6����Һ������
D.��ȫ�к�pH��3��H2SO4��Һ50 mL����ҪpH��11��NaOH��Һ50 mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ĺ������кܶ��֣������������ᡢ�������⣬���кܶ������ᣬ�罹������(H2S2O5)����һ����(H2SO5) ��������(H2S2O8) �ȡ���һ������һ��һԪǿ�ᣬ��������Ӿ����������ṹʽ��ͼ��ʾ������������һ�ְ�ɫ���壬�����ֽ⣬��ǿ��ˮ�ԣ���������ˮ����ˮ�л���ˮ��õ�����������⣬��ش�����������⡣
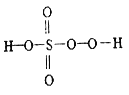
(1)��һ��������Ԫ�صĻ��ϼ���_________����������Ľṹʽ��_____________��
(2)��ҵ���Ʊ�����������Һ������֮һ���£�
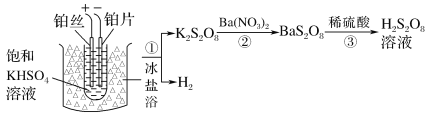
�ٵ��ʱ�����ĵ缫��ӦʽΪ______
�����������ܷ���ͭ˿���沬˿��________(������������������)��˵�����ɣ�__________��
(3)����������(Na2S2O5)����Ҫ�Ŀ�����������ҵ�������̵����е�SO2����Na2S2O5�Ĺ���Ϊ��
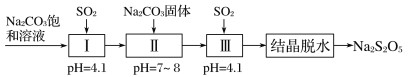
��pH��4.1ʱ������Ϊ________��Һ(д��ѧʽ)��
�ڹ����м���Na2CO3���壬���ٴγ���SO2��Ŀ����___________��
�����ѾƳ���Na2S2O5�������������ڲⶨij���Ѿ���Na2S2O5������ʱ��ȡ50.00 mL���Ѿ���Ʒ����0.010 00 mol��L��1�ĵ��Һ�ζ����յ㣬����10.00 mL���ζ���Ӧ�����ӷ���ʽΪ_______________ ������Ʒ��Na2S2O5�IJ�����Ϊ______g��L��1(��SO2��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ����ʵ��˵��HNO2��������ʵ���
��HNO2��Һ��NaHCO3��Һ��ϣ��ų�����
����HNO2��Һ��������ʵ�飬���ݺܰ�
��HNO2��Һ����Na2SO4��Һ��Ӧ
��0.1mol��L -1HNO2��Һ�У�c(H��)��0.015mol��L-1
����ͬŨ��ʱ��HNO2��Һ�ĵ���������������
��ȡ0.1mol��L -1HNO2��Һ200mL����ˮ�����Ϊ2L��pH>2
A.�٢ڢ�B.�ܢݢ�C.�٢ۢ�D.�٢ܢݢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NaCl��CuSO4������Һ�������Ϻ���ʯī�缫���е�⣬�������У���ҺpH��ʱ��t�仯��������ͼ��ʾ��������˵����ȷ����

A.�������������缫��Ӧ2Cl��-2e��=Cl2����2H��2e����H2��������ͬʱ����
B.�����C��ʱ�����������Һ�м�������CuCl2���壬����ʹ�������Һ�ָ���ԭ����Ũ��
C.AB�α�ʾ��������H������ԭ��pH����
D.ԭ�����Һ��NaCl��CuSO4Ũ��֮��ǡ��Ϊ2:1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£����и���������ָ����Һ���ܴ����������
A.pH��2����Һ��Na+��Fe3+��Cl����NO3-
B.c(NaAlO2)��0.1mol��L��1����Һ��K+��H+��Cl����SO42-
C.c(OH)<![]() ����Һ��Na+��K+��SiO32-��ClO��
����Һ��Na+��K+��SiO32-��ClO��
D.c(Fe3+)��0.1mol��L��1����Һ��Al3+��NO3-��MnO4-��SCN��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I���ݱ������ҹ����Ϻ��������������еĿ�ȼ��(�����ˮ����)�Բɻ�óɹ���������һ����Ҫ�Ļ���ԭ�ϡ�
��1����������������������ʵ���Ҫ��ʽ����������������������֣�
ˮ����������CH4(g)��H2O(g) ![]() CO(g)��3H2(g)����H1����205.9 kJ��mol��1�� ��
CO(g)��3H2(g)����H1����205.9 kJ��mol��1�� ��
CO(g)��H2O(g) ![]() CO2(g)��H2(g)����H2����41.2 kJ��mol��1����
CO2(g)��H2(g)����H2����41.2 kJ��mol��1����
������̼������CH4(g)��CO2(g) ![]() 2CO(g)��2H2(g)����H3����
2CO(g)��2H2(g)����H3����
��Ӧ���Է����е�������______________����H3��________kJ��mol��1��
��.���Ĺ̶�һֱ�ǿ�ѧ���о�����Ҫ���⣬�ϳɰ������˹��̵��Ƚϳ���ļ�������ԭ��ΪN2 (g)��3H2 (g) ![]() 2NH3(g)��
2NH3(g)��
��2���ڲ�ͬ�¶ȡ�ѹǿ����ͬ���������£���ʼN2��H2 �ֱ�Ϊ0.1 mol��0.3 molʱ��ƽ��������а����������(��)����ͼ��ʾ��

�����У�p1��p2 ��p3 �ɴ�С��˳����____________���÷�Ӧ��H _______0(����>����<����������)��
�����ֱ���vA(N2)��vB(N2)��ʾ�ӷ�Ӧ��ʼ����ƽ��״̬A��Bʱ�Ļ�ѧ��Ӧ���ʣ���vA(N2)________vB(N2)(����>����<����������)��
������250 �桢p1 Ϊ105 Pa�����£���Ӧ�ﵽƽ��ʱ���������Ϊ1 L�����������B��N2 �ķ�ѹp(N2)Ϊ_______Pa (��ѹ����ѹ�����ʵ�������������һλС��)��
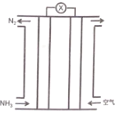
��.�����������(S2O42-)Ϊý�飬ʹ�ü�ӵ绯ѧ��Ҳ�ɴ���ȼú�����е�NO��װ����ͼ��ʾ��

��3�����������ĵ缫��ӦʽΪ___________��
��NO����ת�������Ҫ����ΪNH4+����ͨ��ʱ��·��ת����0.3 mol e�������ͨ����������������յ�NO�ڱ�״���µ����Ϊ________mL��
��.��4�������£���a mol��L-1�Ĵ�����b mol��L-1Ba(OH)2 ��Һ�������ϣ���ַ�Ӧ����Һ�д���2c(Ba2+)=c(CH3COO-)����û����Һ�д���ĵ��볣��Ka=___________(�ú�a��b�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�˵����ȷ����
A. ��֪HI(g) ![]() 1/2H2(g)��1/2I2(s)����H����26.5 kJ��mol��1���ɴ˿�֪1 mol HI�������ܱ������г�ַֽ����Էų�26.5 kJ������
1/2H2(g)��1/2I2(s)����H����26.5 kJ��mol��1���ɴ˿�֪1 mol HI�������ܱ������г�ַֽ����Էų�26.5 kJ������
B. ��֪2H2(g)��O2(g)===2H2O(g)����H����571.6 kJ��mol��1����������ȼ����Ϊ��H����285.8 kJ��mol��1
C. ��֪2C(s)��2O2(g)=2CO2(g) ��H1, 2C(s)��O2(g)=2CO(g) ��H2������H1����H2
D. ��20.0 g NaOH��ϡ��Һ��ϡ������ȫ�кͣ��ų�28.7 kJ����������ϡ�����ϡNaOH��Һ��Ӧ���Ȼ�ѧ����ʽΪ��NaOH(aq)��CH3COOH(aq)===CH3COONa(aq)��H2O(l)����H����57.4 kJ��mol��1
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com